Journal of Fluorine Chemistry ( IF 1.7 ) Pub Date : 2018-05-01 , DOI: 10.1016/j.jfluchem.2018.05.001 Pradeep Kumar Rao , Shridhar P. Gejji
|
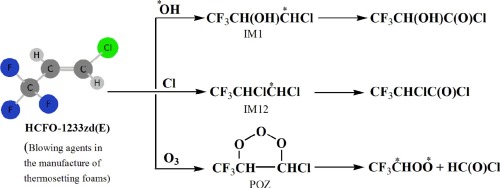
A degradation of trans-CF3CH=CHCl (HCFO-1233zd(E)) by reaction with OH radical, Cl atom and O3 molecule over the temperature range 200–1000 K has been studied employing the density functional theory. The potential energy surfaces accompnying the degradation have further been characterized in terms of single point energies derived from the coupled cluster CCSD(T) theory. Different possible pathways including H-abstraction, addition, isomerisation, dissociation and secondary reactions in absence and presence of O2/NOx are considered to elucidate the underlying mechanisms of the titled reactions. It has been shown that the initiation of degradation proceeds predominantly via addition to olefinic carbon with contributions from the direct H-abstraction channels being negligible as a result of the higher energy barrier. Calculated rate coefficients for the reaction of HCFO-1233zd(E) with
OH was estimated to be 3.2 × 10−13 cm3 molecule‒1 sec‒1 whereas those for the reactions with Cl and O3 are predicted to be 3.7 × 10‒11 cm3 molecule‒1 sec‒1 and 50.0 × 10‒21 cm3 molecule‒1 sec‒1, respectively, which compare well with those from the earlier experiments. The present endeavor describes the atmospheric fate and oxidation processes of titled molecule and provides comprehensive data on the product branching ratios, atmospheric lifetimes and global warming potentials.
中文翻译:

OH自由基,Cl原子和O 3分子引发的HCFO-1233zd(E)的大气降解:动力学,反应机理和意义
利用密度泛函理论,研究了在200–1000 K的温度范围内,与OH自由基,Cl原子和O 3分子反应可降解反式CF 3 CH = CHCl(HCFO-1233zd(E))。伴随退化的势能面已根据耦合簇CCSD(T)理论得出的单点能量进行了进一步表征。在不存在和存在O 2 / NO x的情况下,可能的不同途径包括H吸收,加成,异构化,离解和次级反应被认为是阐明标题反应的潜在机制。已经表明,降解的引发主要是通过向烯烃碳中添加而进行的,由于较高的能垒,来自直接的H吸收通道的贡献可忽略不计。HCFO-1233zd(E)与
OH反应的计算速率系数估计为3.2×10 -13 cm 3 分子‒1 sec ‒1,而与Cl和O 3反应的计算速率系数估计为3.7×10 ‒ 11 cm 3 分子‒1 秒‒1和50.0×10 ‒21 cm 3 分子‒1 秒‒1,分别与早期实验的结果相比。本发明描述标题分子的大气命运和氧化过程,并提供有关产物支化比,大气寿命和全球变暖潜能的综合数据。











































 京公网安备 11010802027423号
京公网安备 11010802027423号