Bioorganic Chemistry ( IF 4.5 ) Pub Date : 2018-05-22 , DOI: 10.1016/j.bioorg.2018.05.021 Muhammad Taha , Mohd Syukri Baharudin , Nor Hadiani Ismail , Syahrul Imran , Muhammad Naseem Khan , Fazal Rahim , Manikandan Selvaraj , Sridevi Chigurupati , Muhammad Nawaz , Faiza Qureshi , Shantini Vijayabalan
|
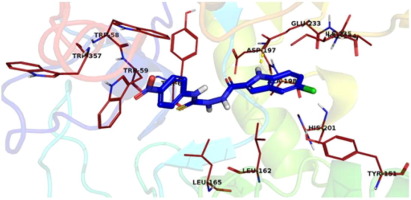
In search of potent α-amylase inhibitor we have synthesized eighteen indole analogs (1–18), characterized by NMR and HR-EIMS and screened for α-amylase inhibitory activity. All analogs exhibited a variable degree of α-amylase inhibition with IC50 values ranging between 2.031 ± 0.11 and 2.633 ± 0.05 μM when compared with standard acarbose having IC50 values 1.927 ± 0.17 μM. All compounds showed good α-amylase inhibition. Compound 14 was found to be the most potent analog among the series. Structure-activity relationship has been established for all compounds mainly based on bringing about the difference of substituents on phenyl ring. To understand the binding interaction of the most active analogs molecular docking study was performed.
中文翻译:

吲哚衍生物的合成,α-淀粉酶抑制潜能及分子对接研究
在搜索有效的α淀粉酶的抑制剂我们合成18个吲哚类似物(1 - 18),其特征在于用NMR和HR-EIMS并筛选α淀粉酶抑制活性。与具有IC 50值为1.927±0.17μM的标准阿卡波糖相比,所有类似物均表现出不同程度的α-淀粉酶抑制作用,IC 50值为2.031±0.11至2.633±0.05μM 。所有化合物均表现出良好的α-淀粉酶抑制作用。化合物14被发现是该系列中最有效的类似物。已经基于引起苯环上取代基的差异而对所有化合物建立了结构-活性关系。为了理解最活跃的类似物的结合相互作用,进行了分子对接研究。











































 京公网安备 11010802027423号
京公网安备 11010802027423号