当前位置:
X-MOL 学术
›
Ind. Eng. Chem. Res.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Spectroscopic Investigation of Enhanced Adsorption of U(VI) and Eu(III) on Magnetic Attapulgite in Binary System
Industrial & Engineering Chemistry Research ( IF 3.8 ) Pub Date : 2018-05-24 , DOI: 10.1021/acs.iecr.8b01803 Yi Xie 1 , Dadong Shao 1 , Xirui Lu 2 , Tasawar Hayat , Njud S. Alharbi , Changlun Chen 1 , Gang Song 3 , Diyun Chen 3 , Yubing Sun 4
Industrial & Engineering Chemistry Research ( IF 3.8 ) Pub Date : 2018-05-24 , DOI: 10.1021/acs.iecr.8b01803 Yi Xie 1 , Dadong Shao 1 , Xirui Lu 2 , Tasawar Hayat , Njud S. Alharbi , Changlun Chen 1 , Gang Song 3 , Diyun Chen 3 , Yubing Sun 4
Affiliation
|
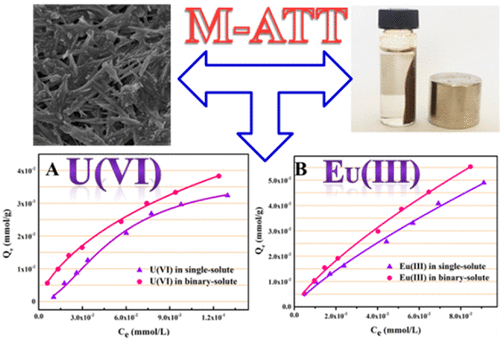
The coadsorption of uranium and europium on magnetic attapulgite (M-ATT) was explored through batch experiments and spectroscopic tests. The adsorption processes of U(VI) and Eu(III) onto M-ATT were highly affected by the solution pH but not by the ionic strength, indicating that the adsorption of the two metals was predominated through inner-sphere surface complexation. Adsorption isotherms demonstrated that the maximum adsorption capacities of U(VI) on M-ATT in the single-solute system (e.g., 60.48 mg g–1 at pH 6.0) were lower than that in the binary-solute system (e.g., 63.03 mg g–1 at pH 6.0), and the same trend was noticed for Eu(III) adsorption due to their synergistic effect. The existence of U(VI) and U(IV) species evidenced the partial reduction of adsorbed U(VI) to U(IV) by M-ATT based on XPS tests. Hence, the enhanced adsorption of U(VI) in the existence of Eu(III) is ascribed to the primary coadsorption and then the redox of adsorbed U(VI) to U(IV) by M-ATT, as well as the formation of new sorbent active sites derived from reductive coprecipitation (e.g., UO2+x(s)), which also increased the Eu(III) adsorption on M-ATT. The findings are significant for coadsorption of radionuclides by magnetic adsorbents in environmental pollution management.
中文翻译:

二元体系中磁性凹凸棒石对U(VI)和Eu(III)增强吸附的光谱研究
通过分批实验和光谱测试探索了铀和euro在凹凸棒石(M-ATT)上的共吸附。U(VI)和Eu(III)在M-ATT上的吸附过程受溶液pH的影响很大,而不受离子强度的影响,表明这两种金属的吸附主要通过内球表面络合作用。吸附等温线表明,单溶质体系中U(VI)在M-ATT上的最大吸附容量(例如,在pH 6.0时为60.48 mg g -1)比二元溶质体系中的最大吸附容量(例如63.03 mg )低g –1在pH 6.0时),由于它们的协同作用,对Eu(III)的吸附也有相同的趋势。U(VI)和U(IV)物种的存在证明了基于XPS测试的M-ATT将吸附的U(VI)部分还原为U(IV)。因此,存在Eu(III)时U(VI)的增强吸附归因于主要共吸附,然后通过M-ATT将吸附的U(VI)还原为U(IV),并形成了还原共沉淀(例如,UO 2+ x(s))产生了新的吸附剂活性位,这也增加了Eu(III)在M-ATT上的吸附。该发现对于环境污染管理中的磁性吸附剂对放射性核素的共吸附具有重要意义。
更新日期:2018-05-25
中文翻译:

二元体系中磁性凹凸棒石对U(VI)和Eu(III)增强吸附的光谱研究
通过分批实验和光谱测试探索了铀和euro在凹凸棒石(M-ATT)上的共吸附。U(VI)和Eu(III)在M-ATT上的吸附过程受溶液pH的影响很大,而不受离子强度的影响,表明这两种金属的吸附主要通过内球表面络合作用。吸附等温线表明,单溶质体系中U(VI)在M-ATT上的最大吸附容量(例如,在pH 6.0时为60.48 mg g -1)比二元溶质体系中的最大吸附容量(例如63.03 mg )低g –1在pH 6.0时),由于它们的协同作用,对Eu(III)的吸附也有相同的趋势。U(VI)和U(IV)物种的存在证明了基于XPS测试的M-ATT将吸附的U(VI)部分还原为U(IV)。因此,存在Eu(III)时U(VI)的增强吸附归因于主要共吸附,然后通过M-ATT将吸附的U(VI)还原为U(IV),并形成了还原共沉淀(例如,UO 2+ x(s))产生了新的吸附剂活性位,这也增加了Eu(III)在M-ATT上的吸附。该发现对于环境污染管理中的磁性吸附剂对放射性核素的共吸附具有重要意义。











































 京公网安备 11010802027423号
京公网安备 11010802027423号