当前位置:
X-MOL 学术
›
Org. Lett.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Synthesis of α,α-Difluorinated Phosphonate pSer/pThr Mimetics via Rhodium-Catalyzed Asymmetric Hydrogenation of β-Difluorophosphonomethyl α-(Acylamino)acrylates
Organic Letters ( IF 4.9 ) Pub Date : 2018-05-21 00:00:00 , DOI: 10.1021/acs.orglett.8b01151 Hong-Xue Chen 1 , Jie Kang 1 , Rong Chang 1 , Yun-Lai Zhang 1 , Hua-Zhen Duan 1 , Yan-Mei Li 1 , Yong-Xiang Chen 1
Organic Letters ( IF 4.9 ) Pub Date : 2018-05-21 00:00:00 , DOI: 10.1021/acs.orglett.8b01151 Hong-Xue Chen 1 , Jie Kang 1 , Rong Chang 1 , Yun-Lai Zhang 1 , Hua-Zhen Duan 1 , Yan-Mei Li 1 , Yong-Xiang Chen 1
Affiliation
|
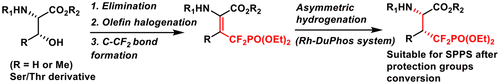
A novel and facile synthetic strategy for α,α-difluorinated phosphonate mimetics of phosphoserine/phosphothreonine utilizing rhodium-catalyzed asymmetric hydrogenation was developed. The dehydrogenated substrate β-difluorophosphonomethyl α-(acylamino)acrylates were first prepared from protected serine/threonine followed by asymmetric hydrogenation using the rhodium–DuPhos catalytic system to generate the chiral center(s). These important phosphonate building blocks were successfully incorporated into phosphatase-resistant peptides, which displayed similar inhibition to the 14-3-3 ζ protein as the parent pSer/pThr peptides.
中文翻译:

β-二氟膦酰基甲基α-(酰基氨基)丙烯酸酯的铑催化不对称加氢合成α,α-二氟膦酸酯pSer / pThr模拟物
提出了利用铑催化的不对称加氢合成磷酸丝氨酸/磷酸苏氨酸的α,α-二氟代膦酸酯模拟物的简便方法。脱氢的底物β-二氟膦酰基甲基α-(酰基氨基)丙烯酸酯首先由受保护的丝氨酸/苏氨酸制备,然后使用铑-DuPhos催化系统进行不对称氢化以生成手性中心。这些重要的膦酸酯结构单元已成功地掺入抗磷酸酶的肽中,与亲本pSer / pThr肽相比,其显示出与14-3-3ζ蛋白相似的抑制作用。
更新日期:2018-05-21
中文翻译:

β-二氟膦酰基甲基α-(酰基氨基)丙烯酸酯的铑催化不对称加氢合成α,α-二氟膦酸酯pSer / pThr模拟物
提出了利用铑催化的不对称加氢合成磷酸丝氨酸/磷酸苏氨酸的α,α-二氟代膦酸酯模拟物的简便方法。脱氢的底物β-二氟膦酰基甲基α-(酰基氨基)丙烯酸酯首先由受保护的丝氨酸/苏氨酸制备,然后使用铑-DuPhos催化系统进行不对称氢化以生成手性中心。这些重要的膦酸酯结构单元已成功地掺入抗磷酸酶的肽中,与亲本pSer / pThr肽相比,其显示出与14-3-3ζ蛋白相似的抑制作用。











































 京公网安备 11010802027423号
京公网安备 11010802027423号