European Journal of Medicinal Chemistry ( IF 6.0 ) Pub Date : 2018-05-21 , DOI: 10.1016/j.ejmech.2018.05.028 Chao-Rui Yang , Bin Peng , Sheng-Li Cao , Ting-Ting Ren , Wei Jiang , Fu-Cheng Wang , You-Shan Li , Guo Wang , Zheng Li , Shibin Xu , Ji Liao , Hailong Wang , Jing Li , Xingzhi Xu
|
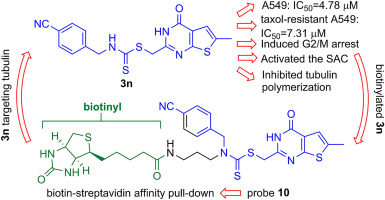
Two series of thieno[2,3-d]pyrimidine derivatives bearing a dithiocarbamate side chain at the C2 position were synthesized and evaluated for cytotoxic activity in human lung cancer A549 and colon cancer HCT-116 cell lines. Compound 3n exhibited the most cytotoxic effect on A549 cells with an IC50 value of 4.87 μM, inducing a cell cycle arrest at G2/M phase and activating the spindle assembly checkpoint (SAC). To identify the target protein(s) of 3n, we incorporated biotin with 3n through a three-carbon chain and an amide bond to synthesize probe 10. The targeted proteins were pulled down from the A549 total cell lysate by biotin-streptavidin affinity purification and analyzed by mass spectrometry. Tubulin was the only protein identified, which is related to the SAC and directly binds to probe 10 both in vivo and in vitro. Furthermore, compound 3n inhibited tubulin polymerization in vitro in a dose-dependent manner, competed with taxol in binding to tubulin, exerting cytotoxic activity toward taxol-resistant A549 cells. These results demonstrate that thieno[2,3-d]pyrimidine derivative 3n exhibits cytotoxicity in cancer cells by targeting tubulin to activate the SAC and potentially acts as a therapeutic lead compound for taxol-resistant cancers.
中文翻译:

在C2位带有二硫代氨基甲酸酯侧链的噻吩并[2,3- d ]嘧啶衍生物的合成,细胞毒性评估和靶标鉴定
合成了两个在C2位置带有二硫代氨基甲酸酯侧链的噻吩并[2,3- d ]嘧啶衍生物,并评估了它们在人肺癌A549和结肠癌HCT-116细胞系中的细胞毒活性。化合物3n对A549细胞表现出最大的细胞毒性作用,IC 50值为4.87μM,诱导细胞周期停滞在G2 / M期并激活纺锤体装配检查点(SAC)。为了鉴定3n的靶蛋白,我们通过三碳链和酰胺键将生物素与3n结合,以合成探针10。通过生物素-链霉亲和素亲和纯化从A549总细胞裂解物中提取靶蛋白,并通过质谱分析。微管蛋白是唯一确定的蛋白质,这是关系到SAC和直接结合到探针10都在体内和体外。此外,化合物3n在体外以剂量依赖性方式抑制微管蛋白聚合,与紫杉醇竞争结合微管蛋白,从而对抗紫杉醇的A549细胞发挥细胞毒活性。这些结果表明噻吩并[2,3- d ]嘧啶衍生物3n 通过靶向微管蛋白激活SAC,在癌细胞中表现出细胞毒性,并有可能作为紫杉醇耐药性癌症的治疗先导化合物。











































 京公网安备 11010802027423号
京公网安备 11010802027423号