European Journal of Medicinal Chemistry ( IF 6.7 ) Pub Date : 2018-05-22 , DOI: 10.1016/j.ejmech.2018.05.029 Florian Braun , Nicole Bertoletti , Gabriele Möller , Jerzy Adamski , Martin Frotscher , Nathalie Guragossian , Patrícia Alexandra Madeira Gírio , Marc Le Borgne , Laurent Ettouati , Pierre Falson , Sebastian Müller , Günther Vollmer , Andreas Heine , Gerhard Klebe , Sandrine Marchais-Oberwinkler
|
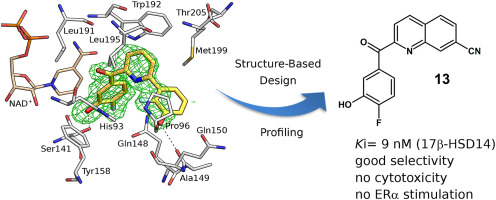
The human enzyme 17β-hydroxysteroid dehydrogenase 14 (17β-HSD14) oxidizes the hydroxyl group at position 17 of estradiol and 5-androstenediol using NAD+ as cofactor. However, the physiological role of the enzyme remains unclear. We recently described the first class of nonsteroidal inhibitors for this enzyme with compound 1 showing a high 17β-HSD14 inhibitory activity. Its crystal structure was used as starting point for a structure-based optimization in this study. The goal was to develop a promising chemical probe to further investigate the enzyme. The newly designed compounds revealed mostly very high inhibition of the enzyme and for seven of them the crystal structures of the corresponding inhibitor-enzyme complexes were resolved. The crystal structures disclosed that a small change in the substitution pattern of the compounds resulted in an alternative binding mode for one inhibitor. The profiling of a set of the most potent inhibitors identified 13 (Ki = 9 nM) with a good selectivity profile toward three 17β-HSDs and the estrogen receptor alpha. This inhibitor displayed no cytotoxicity, good solubility, and auspicious predicted bioavailability. Overall, 13 is a highly interesting 17β-HSD14 inhibitor, which might be used as chemical probe for further investigation of the target enzyme.
中文翻译:

新型17β-HSD14抑制剂的基于结构的设计和分析
人酵素17β-羟基类固醇脱氢酶14(17β-HSD14)使用NAD +作为辅因子,氧化雌二醇和5-雄烯二醇的17位羟基。但是,该酶的生理作用仍不清楚。我们最近与化合物1描述了该酶的第一类非甾体抑制剂表现出很高的17β-HSD14抑制活性。在本研究中,将其晶体结构用作基于结构的优化的起点。目的是开发一种有前途的化学探针,以进一步研究该酶。新设计的化合物大部分显示出对该酶的高度抑制作用,其中有7种的相应抑制剂-酶复合物的晶体结构得以解析。晶体结构表明,化合物取代模式的微小变化会导致一种抑制剂的另一种结合方式。对一组最有效的抑制剂进行了分析,确定了13(K i = 9 nM),对三个17β-HSD和雌激素受体α具有良好的选择性。该抑制剂显示无细胞毒性,良好的溶解性和吉利的预测生物利用度。总的来说,13是一种非常有趣的17β-HSD14抑制剂,可以用作进一步研究目标酶的化学探针。




























 京公网安备 11010802027423号
京公网安备 11010802027423号