当前位置:
X-MOL 学术
›
Org. Chem. Front.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
The serendipitous discovery of a readily available redox-bistable molecule derived from cyclic(alkyl)(amino)carbenes†
Organic Chemistry Frontiers ( IF 5.4 ) Pub Date : 2018-05-21 00:00:00 , DOI: 10.1039/c8qo00447a Janell K. Mahoney 1, 2, 3, 4, 5 , Vianney Regnier 6, 7, 8, 9, 10 , Erik A. Romero 1, 2, 3, 4, 5 , Florian Molton 6, 7, 8, 9, 10 , Guy Royal 6, 7, 8, 9, 10 , Rodolphe Jazzar 1, 2, 3, 4, 5 , David Martin 6, 7, 8, 9, 10 , Guy Bertrand 1, 2, 3, 4, 5
Organic Chemistry Frontiers ( IF 5.4 ) Pub Date : 2018-05-21 00:00:00 , DOI: 10.1039/c8qo00447a Janell K. Mahoney 1, 2, 3, 4, 5 , Vianney Regnier 6, 7, 8, 9, 10 , Erik A. Romero 1, 2, 3, 4, 5 , Florian Molton 6, 7, 8, 9, 10 , Guy Royal 6, 7, 8, 9, 10 , Rodolphe Jazzar 1, 2, 3, 4, 5 , David Martin 6, 7, 8, 9, 10 , Guy Bertrand 1, 2, 3, 4, 5
Affiliation
|
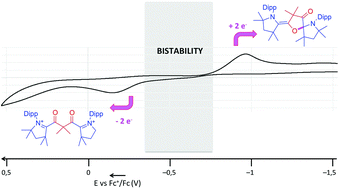
Bis(azoliums) are readily available in one step from cyclic (alkyl)(amino)carbenes and bis(acyl chlorides). A two-electron reduction of the bis(azolium), featuring a gem-(dimethyl)malonoyl spacer, leads to the corresponding transient diradical, which undergoes an intramolecular cyclization. The latter can be re-oxidized at a higher potential to yield back the bis(azolium). The redox bistability of this simple organic molecular system is linked to the formation of a weak C–O bond (27 kcal mol−1). Both redox forms can be isolated and stored for months without evidence of decay.
中文翻译:

偶然发现了衍生自环状(烷基)(氨基)卡宾的氧化还原双稳态分子†
在一个步骤中,双(azo)很容易从环状(烷基)(氨基)卡宾和双(酰氯)中获得。以宝石-(二甲基)丙二酰基间隔基为特征的双(偶氮)的双电子还原导致相应的瞬态双自由基,其经历分子内环化。后者可以在较高电势下被重新氧化,以产生双(偶氮)。这种简单的有机分子系统的氧化还原双稳态性与弱C–O键(27 kcal mol -1)的形成有关。两种氧化还原形式都可以分离并保存数月,而没有衰减的迹象。
更新日期:2018-05-21
中文翻译:

偶然发现了衍生自环状(烷基)(氨基)卡宾的氧化还原双稳态分子†
在一个步骤中,双(azo)很容易从环状(烷基)(氨基)卡宾和双(酰氯)中获得。以宝石-(二甲基)丙二酰基间隔基为特征的双(偶氮)的双电子还原导致相应的瞬态双自由基,其经历分子内环化。后者可以在较高电势下被重新氧化,以产生双(偶氮)。这种简单的有机分子系统的氧化还原双稳态性与弱C–O键(27 kcal mol -1)的形成有关。两种氧化还原形式都可以分离并保存数月,而没有衰减的迹象。



























 京公网安备 11010802027423号
京公网安备 11010802027423号