当前位置:
X-MOL 学术
›
Chem. Sci.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Carboxylate-directed C–H allylation with allyl alcohols or ethers†
Chemical Science ( IF 7.6 ) Pub Date : 2018-05-21 00:00:00 , DOI: 10.1039/c8sc01741g Xiao-Qiang Hu 1 , Zhiyong Hu 1 , A Stefania Trita 1 , Guodong Zhang 1 , Lukas J Gooßen 1
Chemical Science ( IF 7.6 ) Pub Date : 2018-05-21 00:00:00 , DOI: 10.1039/c8sc01741g Xiao-Qiang Hu 1 , Zhiyong Hu 1 , A Stefania Trita 1 , Guodong Zhang 1 , Lukas J Gooßen 1
Affiliation
|
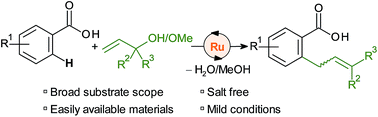
A [Ru(p-cymene)Cl2]2 catalyst activates allyl alcohols and ethers for the regioselective ortho-C–H allylation of aromatic and heteroaromatic carboxylates. The reaction is orthogonal to most C–H functionalisations with allyl alcohols in that allyl arenes rather than carbonyl compounds are obtained. A wide range of substrates are thus smoothly transformed to allylarenes at 50 °C in phosphate-buffered 2,2,2-trichloroethanol. The reaction concept combines the use of abundant reagents and directing groups in a sustainable, waste-minimised method for C–C bond formation.
中文翻译:

用烯丙醇或醚进行羧酸盐引导的 C–H 烯丙基化†
[Ru( p-伞花烃)Cl 2 ] 2催化剂可活化烯丙醇和醚,以实现芳香族和杂芳香族羧酸酯的区域选择性邻位-C–H烯丙基化。该反应与大多数烯丙醇的 C-H 官能化反应正交,因为获得的是烯丙基芳烃而不是羰基化合物。因此,在 50 °C 磷酸盐缓冲的 2,2,2-三氯乙醇中,多种底物可顺利转化为烯丙芳类。该反应概念结合了丰富的试剂和导向基团的使用,以可持续、废物最小化的方法形成 C-C 键。
更新日期:2018-05-21
中文翻译:

用烯丙醇或醚进行羧酸盐引导的 C–H 烯丙基化†
[Ru( p-伞花烃)Cl 2 ] 2催化剂可活化烯丙醇和醚,以实现芳香族和杂芳香族羧酸酯的区域选择性邻位-C–H烯丙基化。该反应与大多数烯丙醇的 C-H 官能化反应正交,因为获得的是烯丙基芳烃而不是羰基化合物。因此,在 50 °C 磷酸盐缓冲的 2,2,2-三氯乙醇中,多种底物可顺利转化为烯丙芳类。该反应概念结合了丰富的试剂和导向基团的使用,以可持续、废物最小化的方法形成 C-C 键。











































 京公网安备 11010802027423号
京公网安备 11010802027423号