当前位置:
X-MOL 学术
›
Asian J. Org. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
BF3‐Catalyzed Synthesis of Cyclic Carbamates from Boc‐Protected Aminals and Alkynes
Asian Journal of Organic Chemistry ( IF 2.8 ) Pub Date : 2018-06-14 , DOI: 10.1002/ajoc.201800289 Taichi Kano 1 , Kento Yasumoto 1 , Keiji Maruoka 1, 2
Asian Journal of Organic Chemistry ( IF 2.8 ) Pub Date : 2018-06-14 , DOI: 10.1002/ajoc.201800289 Taichi Kano 1 , Kento Yasumoto 1 , Keiji Maruoka 1, 2
Affiliation
|
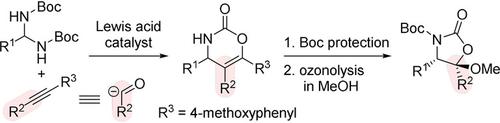
Cyclic carbamates were synthesized by the acid‐catalyzed reaction between Boc‐protected aminals as an imine precursor and 4‐methoxyphenyl‐substituted alkynes. The resulting cyclic carbamates could be converted to the one carbon ring‐contracted cyclic carbamates by ozonolysis. Consequently, 4‐methoxyphenyl‐substituted alkynes served as masked acyl anion equivalents.
中文翻译:

BF3催化从Boc保护的缩醛和炔烃中合成环状氨基甲酸酯
环状氨基甲酸酯是通过Boc保护的作为亚胺前体的氨基缩醛与4-甲氧基苯基取代的炔烃之间的酸催化反应合成的。所产生的环状氨基甲酸酯可通过臭氧分解转化为一个碳环收缩的环状氨基甲酸酯。因此,4-甲氧基苯基取代的炔烃可作为掩蔽的酰基阴离子等价物。
更新日期:2018-06-14
中文翻译:

BF3催化从Boc保护的缩醛和炔烃中合成环状氨基甲酸酯
环状氨基甲酸酯是通过Boc保护的作为亚胺前体的氨基缩醛与4-甲氧基苯基取代的炔烃之间的酸催化反应合成的。所产生的环状氨基甲酸酯可通过臭氧分解转化为一个碳环收缩的环状氨基甲酸酯。因此,4-甲氧基苯基取代的炔烃可作为掩蔽的酰基阴离子等价物。











































 京公网安备 11010802027423号
京公网安备 11010802027423号