当前位置:
X-MOL 学术
›
Angew. Chem. Int. Ed.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Iridium-Catalyzed, Silyl-Directed, peri-Borylation of C-H Bonds in Fused Polycyclic Arenes and Heteroarenes.
Angewandte Chemie International Edition ( IF 16.1 ) Pub Date : 2018-07-13 , DOI: 10.1002/anie.201805086 Bo Su 1 , John F Hartwig 1
Angewandte Chemie International Edition ( IF 16.1 ) Pub Date : 2018-07-13 , DOI: 10.1002/anie.201805086 Bo Su 1 , John F Hartwig 1
Affiliation
|
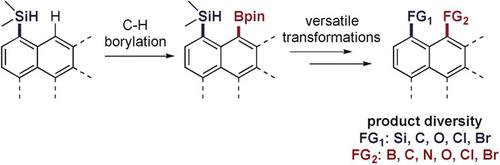
peri‐Disubstituted naphthalenes exhibit interesting physical properties and unique chemical reactivity, due to the parallel arrangement of the bonds to the two peri‐disposed substituents. Regioselective installation of a functional group at the position peri to 1‐substituted naphthalenes is challenging due to the steric interaction between the existing substituent and the position at which the second one would be installed. We report an iridium‐catalyzed borylation of the C−H bond peri to a silyl group in naphthalenes and analogous polyaromatic hydrocarbons. The reaction occurs under mild conditions with wide functional group tolerance. The silyl group and the boryl group in the resulting products are precursors to a range of functional groups bound to the naphthalene ring through C−C, C−O, C−N, C−Br and C−Cl bonds.
中文翻译:

铱催化,甲硅烷基定向,熔融多环芳烃和杂芳烃中CH键的环芳基化。
围二取代的萘表现出令人感兴趣的物理性质和独特的化学反应性,由于粘结到两个平行排列围-disposed取代基。由于在现有取代基和第二个取代基的安装位置之间存在空间相互作用,因此将官能团区域选择性地安装在1取代的萘周围的位置具有挑战性。我们报告了铱键催化的CH键周围的硼化萘和类似的聚芳烃中的甲硅烷基。反应在温和的条件下进行,具有宽泛的官能团耐受性。所得产物中的甲硅烷基和硼烷基是通过CC,CO,CN,CN,Br和CCl键与萘环键合的一系列官能团的前体。
更新日期:2018-07-13
中文翻译:

铱催化,甲硅烷基定向,熔融多环芳烃和杂芳烃中CH键的环芳基化。
围二取代的萘表现出令人感兴趣的物理性质和独特的化学反应性,由于粘结到两个平行排列围-disposed取代基。由于在现有取代基和第二个取代基的安装位置之间存在空间相互作用,因此将官能团区域选择性地安装在1取代的萘周围的位置具有挑战性。我们报告了铱键催化的CH键周围的硼化萘和类似的聚芳烃中的甲硅烷基。反应在温和的条件下进行,具有宽泛的官能团耐受性。所得产物中的甲硅烷基和硼烷基是通过CC,CO,CN,CN,Br和CCl键与萘环键合的一系列官能团的前体。











































 京公网安备 11010802027423号
京公网安备 11010802027423号