当前位置:
X-MOL 学术
›
Chem. Rev.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Multiple Architectures and Mechanisms of Latency in Metallopeptidase Zymogens
Chemical Reviews ( IF 51.4 ) Pub Date : 2018-05-18 00:00:00 , DOI: 10.1021/acs.chemrev.8b00030 Joan L. Arolas 1 , Theodoros Goulas 1 , Anna Cuppari 1 , F. Xavier Gomis-Rüth 1
Chemical Reviews ( IF 51.4 ) Pub Date : 2018-05-18 00:00:00 , DOI: 10.1021/acs.chemrev.8b00030 Joan L. Arolas 1 , Theodoros Goulas 1 , Anna Cuppari 1 , F. Xavier Gomis-Rüth 1
Affiliation
|
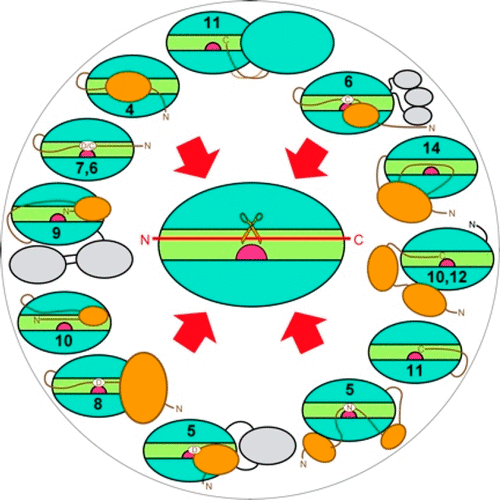
Metallopeptidases cleave polypeptides bound in the active-site cleft of catalytic domains through a general base/acid mechanism. This involves a solvent molecule bound to a catalytic zinc and general regulation of the mechanism through zymogen-based latency. Sixty reported structures from 11 metallopeptidase families reveal that prosegments, mostly N-terminal of the catalytic domain, block the cleft regardless of their size. Prosegments may be peptides (5–14 residues), which are only structured within the zymogens, or large moieties (<227 residues) of one or two folded domains. While some prosegments globally shield the catalytic domain through a few contacts, others specifically run across the cleft in the same or opposite direction as a substrate, making numerous interactions. Some prosegments block the zinc by replacing the solvent with particular side chains, while others use terminal α-amino or carboxylate groups. Overall, metallopeptidase zymogens employ disparate mechanisms that diverge even within families, which supports that latency is less conserved than catalysis.
中文翻译:

Metallopeptidase Zymogens的延迟的多种体系结构和机制。
金属肽酶通过一般的碱/酸机制切割结合在催化结构域的活性位点裂隙中的多肽。这涉及与催化锌结合的溶剂分子,以及通过基于酶原的潜伏期对该机制的一般调节。来自11个金属肽酶家族的60个报告的结构表明,无论大小如何,前段大部分都位于催化结构域的N末端,可阻断裂隙。前体可能是仅在酶原内结构化的肽(5-14个残基),也可能是一个或两个折叠域的大部分(<227个残基)。尽管某些部分通过一些接触而整体上遮蔽了催化区域,但其他部分则以与底物相同或相反的方向穿过裂隙,从而产生了许多相互作用。一些部分通过用特定的侧链取代溶剂来封闭锌,而其他部分则使用末端α-氨基或羧酸酯基团。总体而言,金属肽酶的酶原采用不同的机制,甚至在家族中也存在差异,这支持了等待时间比催化作用更不保守。
更新日期:2018-05-18
中文翻译:

Metallopeptidase Zymogens的延迟的多种体系结构和机制。
金属肽酶通过一般的碱/酸机制切割结合在催化结构域的活性位点裂隙中的多肽。这涉及与催化锌结合的溶剂分子,以及通过基于酶原的潜伏期对该机制的一般调节。来自11个金属肽酶家族的60个报告的结构表明,无论大小如何,前段大部分都位于催化结构域的N末端,可阻断裂隙。前体可能是仅在酶原内结构化的肽(5-14个残基),也可能是一个或两个折叠域的大部分(<227个残基)。尽管某些部分通过一些接触而整体上遮蔽了催化区域,但其他部分则以与底物相同或相反的方向穿过裂隙,从而产生了许多相互作用。一些部分通过用特定的侧链取代溶剂来封闭锌,而其他部分则使用末端α-氨基或羧酸酯基团。总体而言,金属肽酶的酶原采用不同的机制,甚至在家族中也存在差异,这支持了等待时间比催化作用更不保守。











































 京公网安备 11010802027423号
京公网安备 11010802027423号