当前位置:
X-MOL 学术
›
Ind. Eng. Chem. Res.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Ultrafast, Continuous and Shape-Controlled Preparation of CeO2 Nanostructures: Nanorods and Nanocubes in a Microfluidic System
Industrial & Engineering Chemistry Research ( IF 3.8 ) Pub Date : 2018-05-25 , DOI: 10.1021/acs.iecr.8b01298 Hongbao Yao 1 , Yujun Wang 1 , Yu Jing 1 , Guangsheng Luo 1
Industrial & Engineering Chemistry Research ( IF 3.8 ) Pub Date : 2018-05-25 , DOI: 10.1021/acs.iecr.8b01298 Hongbao Yao 1 , Yujun Wang 1 , Yu Jing 1 , Guangsheng Luo 1
Affiliation
|
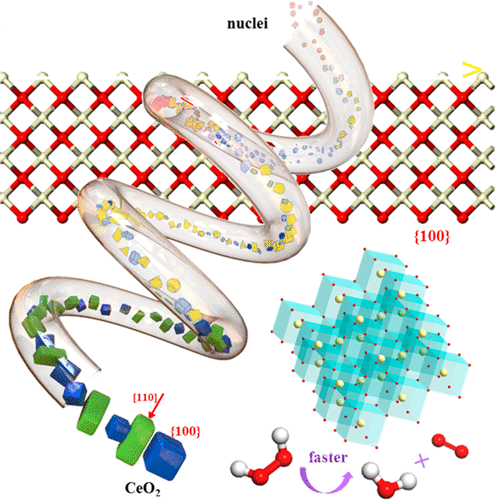
Ceria nanorods enclosed with {110}/{100} planes and ceria nanocubes with {100} planes have been successfully prepared using a homemade microfluidic system in a continuous, ultrafast and shape-controllable manner. Only 8 min of reaction time are needed rather than days to synthesize ceria nanostructures in the traditional batch hydrothermal method. During the synthesis, reaction temperatures and base concentration have been demonstrated as the key factors responsible for the shape evolution. Accordingly, a morphological phase diagram was determined. In addition, polyvinylpyrrolidone was introduced to realize the transformation from ceria nanorods to nanocubes under unfavorable hydrothermal conditions. Catalytic performance of different CeO2 architectures was also examined in decomposing hydrogen peroxide and a reactivity trend (nanoparticles < nanorods < nonocubes) was observed. This is assumed to be related with different surface oxygen vacancy amounts as a result of {110}/{100} preferential planes, as confirmed by X-ray photoelectron spectroscopy and Raman analyses as well as density functional theory (DFT+U) calculations.
中文翻译:

CeO 2纳米结构的超快,连续和形状控制制备:微流体系统中的纳米棒和纳米立方体
用自制的微流体系统以连续,超快和形状可控的方式成功制备了用{110} / {100}平面包围的二氧化铈纳米棒和具有{100}平面的二氧化铈纳米立方体。在传统的间歇水热法中,仅需要8分钟的反应时间即可合成二氧化铈纳米结构,而不是几天。在合成过程中,反应温度和碱浓度已被证明是导致形状演变的关键因素。因此,确定了形态相图。另外,引入聚乙烯吡咯烷酮以在不利的水热条件下实现从二氧化铈纳米棒到纳米立方体的转化。不同CeO 2的催化性能还检查了过氧化氢分解的体系结构,并观察到了反应性趋势(纳米颗粒<纳米棒<非立方体)。X射线光电子能谱和拉曼分析以及密度泛函理论(DFT + U)的计算证实,这是由于{110} / {100}优先平面而导致的表面氧空位不同。
更新日期:2018-05-25
中文翻译:

CeO 2纳米结构的超快,连续和形状控制制备:微流体系统中的纳米棒和纳米立方体
用自制的微流体系统以连续,超快和形状可控的方式成功制备了用{110} / {100}平面包围的二氧化铈纳米棒和具有{100}平面的二氧化铈纳米立方体。在传统的间歇水热法中,仅需要8分钟的反应时间即可合成二氧化铈纳米结构,而不是几天。在合成过程中,反应温度和碱浓度已被证明是导致形状演变的关键因素。因此,确定了形态相图。另外,引入聚乙烯吡咯烷酮以在不利的水热条件下实现从二氧化铈纳米棒到纳米立方体的转化。不同CeO 2的催化性能还检查了过氧化氢分解的体系结构,并观察到了反应性趋势(纳米颗粒<纳米棒<非立方体)。X射线光电子能谱和拉曼分析以及密度泛函理论(DFT + U)的计算证实,这是由于{110} / {100}优先平面而导致的表面氧空位不同。









































 京公网安备 11010802027423号
京公网安备 11010802027423号