当前位置:
X-MOL 学术
›
ACS Infect. Dis.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Characterization of Novel Piperidine-Based Inhibitor of Cathepsin B-Dependent Bacterial Toxins and Viruses
ACS Infectious Diseases ( IF 4.0 ) Pub Date : 2018-05-11 00:00:00 , DOI: 10.1021/acsinfecdis.8b00053 Stella Hartmann 1 , Renae Lopez Cruz 2 , Saleem Alameh 1 , Chi-Lee C. Ho 2 , Amy Rabideau 3 , Bradley L. Pentelute 3 , Kenneth A. Bradley 2 , Mikhail Martchenko 1
ACS Infectious Diseases ( IF 4.0 ) Pub Date : 2018-05-11 00:00:00 , DOI: 10.1021/acsinfecdis.8b00053 Stella Hartmann 1 , Renae Lopez Cruz 2 , Saleem Alameh 1 , Chi-Lee C. Ho 2 , Amy Rabideau 3 , Bradley L. Pentelute 3 , Kenneth A. Bradley 2 , Mikhail Martchenko 1
Affiliation
|
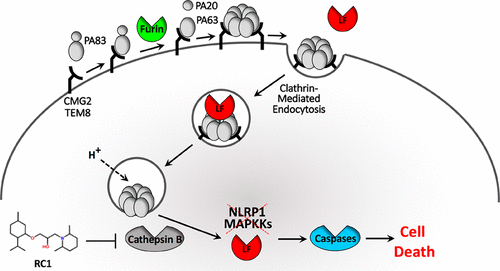
Exploiting the host endocytic trafficking pathway is a common mechanism by which bacterial exotoxins gain entry to exert virulent effects upon the host cells. A previous study identified a small-molecule, 1-(2,6-dimethyl-1-piperidinyl)-3-[(2-isopropyl-5-methylcyclohexyl)oxy]-2-propanol, that blocks the process of anthrax lethal toxin (LT) cytotoxicity. Here, we report the characterization of the bioactivity of this compound, which we named RC1. We found that RC1 protected host cells independently of LT concentration and also blocked intoxication by other bacterial exotoxins, suggesting that the target of the compound is a host factor. Using the anthrax LT intoxication pathway as a reference, we show that while anthrax toxin is able to bind to cells and establish an endosomal pore in the presence of the drug, the toxin is unable to translocate into the cytosol. We demonstrate that RC1 does not inhibit the toxin directly but rather reduces the enzymatic activity of host cathepsin B that mediates the escape of toxins into the cytoplasm from late endosomes. We demonstrate that the pathogenicity of Human cytomegalovirus and Herpes simplex virus 1, which relies on cathepsin B protease activity, is reduced by RC1. This study reveals the potential of RC1 as a broad-spectrum host-oriented therapy against several aggressive and deadly pathogens.
中文翻译:

新型基于哌啶的组织蛋白酶B依赖性细菌毒素和病毒抑制剂的表征
利用宿主的内吞运输途径是细菌外毒素进入的常见机制,可对宿主细胞产生有毒作用。先前的研究确定了一种小分子1-(2,6-二甲基-1-哌啶基)-3-[(2-异丙基-5-甲基环己基)氧基] -2-丙醇可阻止炭疽致死毒素的产生(LT)细胞毒性。在这里,我们报告了该化合物的生物活性的表征,我们将其命名为RC1。我们发现RC1独立于LT浓度保护了宿主细胞,并且还阻止了其他细菌外毒素的中毒作用,这表明该化合物的靶标是宿主因子。以炭疽LT中毒途径为参考,我们显示,尽管炭疽毒素能够与细胞结合并在药物存在下建立内体孔,毒素无法转运到细胞质中。我们证明RC1不会直接抑制毒素,而是降低宿主组织蛋白酶B的酶促活性,该酶介导毒素从晚期内体进入细胞质。我们证明了人类巨细胞病毒和单纯疱疹病毒1的致病性,依赖于组织蛋白酶B蛋白酶活性,被RC1降低。这项研究揭示了RC1作为针对多种侵袭性和致命性病原体的广谱宿主导向疗法的潜力。依赖于组织蛋白酶B蛋白酶活性的分子被RC1降低。这项研究揭示了RC1作为针对多种侵袭性和致命性病原体的广谱宿主导向疗法的潜力。依赖于组织蛋白酶B蛋白酶活性的分子被RC1降低。这项研究揭示了RC1作为针对多种侵袭性和致命性病原体的广谱宿主导向疗法的潜力。
更新日期:2018-05-11
中文翻译:

新型基于哌啶的组织蛋白酶B依赖性细菌毒素和病毒抑制剂的表征
利用宿主的内吞运输途径是细菌外毒素进入的常见机制,可对宿主细胞产生有毒作用。先前的研究确定了一种小分子1-(2,6-二甲基-1-哌啶基)-3-[(2-异丙基-5-甲基环己基)氧基] -2-丙醇可阻止炭疽致死毒素的产生(LT)细胞毒性。在这里,我们报告了该化合物的生物活性的表征,我们将其命名为RC1。我们发现RC1独立于LT浓度保护了宿主细胞,并且还阻止了其他细菌外毒素的中毒作用,这表明该化合物的靶标是宿主因子。以炭疽LT中毒途径为参考,我们显示,尽管炭疽毒素能够与细胞结合并在药物存在下建立内体孔,毒素无法转运到细胞质中。我们证明RC1不会直接抑制毒素,而是降低宿主组织蛋白酶B的酶促活性,该酶介导毒素从晚期内体进入细胞质。我们证明了人类巨细胞病毒和单纯疱疹病毒1的致病性,依赖于组织蛋白酶B蛋白酶活性,被RC1降低。这项研究揭示了RC1作为针对多种侵袭性和致命性病原体的广谱宿主导向疗法的潜力。依赖于组织蛋白酶B蛋白酶活性的分子被RC1降低。这项研究揭示了RC1作为针对多种侵袭性和致命性病原体的广谱宿主导向疗法的潜力。依赖于组织蛋白酶B蛋白酶活性的分子被RC1降低。这项研究揭示了RC1作为针对多种侵袭性和致命性病原体的广谱宿主导向疗法的潜力。











































 京公网安备 11010802027423号
京公网安备 11010802027423号