当前位置:
X-MOL 学术
›
Angew. Chem. Int. Ed.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Cleavage of Unactivated Si−C(sp3) Bonds with Reed's Carborane Acids: Formation of Known and Unknown Silylium Ions
Angewandte Chemie International Edition ( IF 16.1 ) Pub Date : 2018-06-19 , DOI: 10.1002/anie.201805637 Qian Wu 1 , Zheng-Wang Qu 2 , Lukas Omann 1 , Elisabeth Irran 1 , Hendrik F. T. Klare 1 , Martin Oestreich 1
Angewandte Chemie International Edition ( IF 16.1 ) Pub Date : 2018-06-19 , DOI: 10.1002/anie.201805637 Qian Wu 1 , Zheng-Wang Qu 2 , Lukas Omann 1 , Elisabeth Irran 1 , Hendrik F. T. Klare 1 , Martin Oestreich 1
Affiliation
|
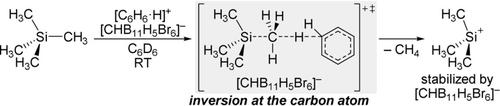
An efficient method for the benzenium‐ion‐mediated cleavage of inert Si−C(sp3) bonds is reported. Various tetraalkylsilanes can thus be converted into the corresponding counteranion‐stabilized silylium ions. The reaction is chemoselective in the case of hexamethyldisilane. Computations reveal a mechanism with backside attack of the proton at one of the alkyl groups. Several activated Si−C(spn) bonds (n=3–1) react equally well, and the procedure can be extended to the generation of stannylium ions.
中文翻译:

未活化的Si-C(sp3)键与里德的硼烷酸的裂解:已知和未知的硅离子的形成
据报道,一种有效的方法可用于苯离子介导的惰性Si-C(sp 3)键的裂解。因此,各种四烷基硅烷可转化为相应的抗衡阴离子稳定的甲硅烷基离子。在六甲基乙硅烷的情况下,该反应是化学选择性的。计算揭示了质子在一个烷基上具有背面进攻的机理。几个活化的Si-C(sp n)键(n = 3-1)反应同样好,该过程可以扩展到生成苯乙烯离子。
更新日期:2018-06-19
中文翻译:

未活化的Si-C(sp3)键与里德的硼烷酸的裂解:已知和未知的硅离子的形成
据报道,一种有效的方法可用于苯离子介导的惰性Si-C(sp 3)键的裂解。因此,各种四烷基硅烷可转化为相应的抗衡阴离子稳定的甲硅烷基离子。在六甲基乙硅烷的情况下,该反应是化学选择性的。计算揭示了质子在一个烷基上具有背面进攻的机理。几个活化的Si-C(sp n)键(n = 3-1)反应同样好,该过程可以扩展到生成苯乙烯离子。











































 京公网安备 11010802027423号
京公网安备 11010802027423号