当前位置:
X-MOL 学术
›
J. Phys. Chem. A
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Mechanistic Study on Water Splitting Reactions by Small Silicon Clusters Si3X, X = Si, Be, Mg, Ca
The Journal of Physical Chemistry A ( IF 2.7 ) Pub Date : 2018-05-18 00:00:00 , DOI: 10.1021/acs.jpca.8b02237 Tran Dieu Hang 1, 2 , Minh Tho Nguyen 2, 3
The Journal of Physical Chemistry A ( IF 2.7 ) Pub Date : 2018-05-18 00:00:00 , DOI: 10.1021/acs.jpca.8b02237 Tran Dieu Hang 1, 2 , Minh Tho Nguyen 2, 3
Affiliation
|
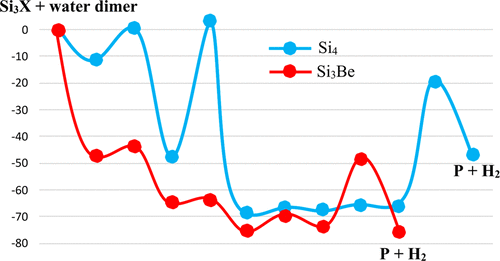
Interaction, dissociation, and dehydrogenation reactions of water monomer and dimer with pure and mixed tetrameric silicon clusters Si3X with X = Si, Be, Mg, Ca were investigated using high accuracy quantum chemical calculations. While geometries were optimized using the DFT/B3LYP functional with the aug-cc-pVTZ basis set, reaction energy profiles were constructed making use of the coupled-cluster theory with extrapolation to complete basis set, CCSD(T)/CBS. Cleavage of the O–H bond in water dimer is found to be more favored than that of water monomer in the reaction with Si4. The water acceptor monomer in water dimer performs as an internal catalyst facilitating H atom transfer to form H2. Adsorption of water dimer on Si3X clusters mostly takes place upon interaction of the donor water molecule with Si cluster. Water dimer adsorbs more strongly on Si3M than on Si4. The most stable complexes obtained upon interaction of water dimer with Si3M mainly arise from M–O interaction in preference over a Si–O connection. Substitution of a Si atom in Si4 by an earth alkaline metal induces a substantial reduction of the energy barrier for the (rate-limiting) first O–H bond cleavage of water dimer. The most remarkable achievement upon doping is a disappearance of the overall energy barrier for the initial O–H bond cleavage in water dimer. Of the three binary Si3M clusters considered, dehydrogenation of water dimer driven by Si3Be is the most kinetically and thermodynamically favorable pathway. In comparison to another cluster such as Al6 and nanoparticles Ru55, energy barriers for water dimer dissociation on Si3M are much lower. The mixed clusters Si3M turn out to be as efficient alternative reagents for O–H dissociation and hydrogen production from water dimer. This study proposes further searches for other mixed silicon clusters as realistic gas phase reagents for crucial dehydrogenation processes in such a way they can be prepared and conducted in experiment.
中文翻译:

Si 3 X,X = Si,Be,Mg,Ca的小硅团簇的水分解反应机理研究
使用高精度量子化学计算研究了水单体和二聚体与纯和混合的四聚硅团簇Si 3 X的相互作用,离解和脱氢反应,其中X = Si,Be,Mg,Ca。虽然使用DFT / B3LYP函数和aug-cc-pVTZ基础集优化了几何结构,但利用耦合簇理论和外推法构建了完整的基础集CCSD(T)/ CBS,从而构建了反应能曲线。在与Si 4的反应中,发现水二聚体中O-H键的裂解比水单体更易裂解。水二聚体中的水受体单体起促进H原子转移形成H 2的内部催化剂的作用。水二聚体在Si 3上的吸附X团簇主要在供体水分子与Si团簇相互作用时发生。水二聚体在Si 3 M上的吸附比在Si 4上的吸附强。水二聚体与Si 3 M相互作用时获得的最稳定的络合物主要来自M-O相互作用,而不是Si-O连接。碱土金属取代Si 4中的Si原子会导致(限速)水二聚体第一次(氢)键断裂的能垒大大降低。掺杂时最显着的成就是消失了二聚体中最初的O-H键断裂的总能垒。在考虑的三个二元Si 3 M团簇中,由Si驱动的水二聚体脱氢3 Be是在动力学和热力学上最有利的途径。与另一个簇(例如Al 6和纳米颗粒Ru 55)相比,Si 3 M上水二聚体解离的能垒要低得多。事实证明,混合簇Si 3 M可作为O-H分解和水二聚体产氢的有效替代试剂。这项研究提出了对其他混合硅团簇的进一步研究,这些簇团对于关键的脱氢过程来说是可行的气相试剂,因此可以在实验中制备和进行。
更新日期:2018-05-18
中文翻译:

Si 3 X,X = Si,Be,Mg,Ca的小硅团簇的水分解反应机理研究
使用高精度量子化学计算研究了水单体和二聚体与纯和混合的四聚硅团簇Si 3 X的相互作用,离解和脱氢反应,其中X = Si,Be,Mg,Ca。虽然使用DFT / B3LYP函数和aug-cc-pVTZ基础集优化了几何结构,但利用耦合簇理论和外推法构建了完整的基础集CCSD(T)/ CBS,从而构建了反应能曲线。在与Si 4的反应中,发现水二聚体中O-H键的裂解比水单体更易裂解。水二聚体中的水受体单体起促进H原子转移形成H 2的内部催化剂的作用。水二聚体在Si 3上的吸附X团簇主要在供体水分子与Si团簇相互作用时发生。水二聚体在Si 3 M上的吸附比在Si 4上的吸附强。水二聚体与Si 3 M相互作用时获得的最稳定的络合物主要来自M-O相互作用,而不是Si-O连接。碱土金属取代Si 4中的Si原子会导致(限速)水二聚体第一次(氢)键断裂的能垒大大降低。掺杂时最显着的成就是消失了二聚体中最初的O-H键断裂的总能垒。在考虑的三个二元Si 3 M团簇中,由Si驱动的水二聚体脱氢3 Be是在动力学和热力学上最有利的途径。与另一个簇(例如Al 6和纳米颗粒Ru 55)相比,Si 3 M上水二聚体解离的能垒要低得多。事实证明,混合簇Si 3 M可作为O-H分解和水二聚体产氢的有效替代试剂。这项研究提出了对其他混合硅团簇的进一步研究,这些簇团对于关键的脱氢过程来说是可行的气相试剂,因此可以在实验中制备和进行。









































 京公网安备 11010802027423号
京公网安备 11010802027423号