当前位置:
X-MOL 学术
›
J. Phys. Chem. A
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Near-Infrared Fluorescence from In-Plane-Aromatic Cycloparaphenylene Dications
The Journal of Physical Chemistry A ( IF 2.7 ) Pub Date : 2018-05-18 00:00:00 , DOI: 10.1021/acs.jpca.8b03105 Yui Masumoto 1, 2 , Naoyuki Toriumi 1 , Atsuya Muranaka 2 , Eiichi Kayahara 3 , Shigeru Yamago 3 , Masanobu Uchiyama 1, 2
The Journal of Physical Chemistry A ( IF 2.7 ) Pub Date : 2018-05-18 00:00:00 , DOI: 10.1021/acs.jpca.8b03105 Yui Masumoto 1, 2 , Naoyuki Toriumi 1 , Atsuya Muranaka 2 , Eiichi Kayahara 3 , Shigeru Yamago 3 , Masanobu Uchiyama 1, 2
Affiliation
|
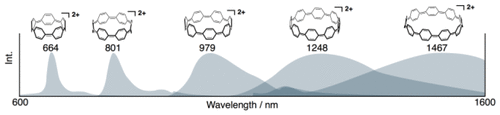
Cycloparaphenylenes (CPPs) are hoop-shaped conjugated hydrocarbons corresponding to partial structures of fullerenes or armchair carbon nanotubes. Here, we examined the fluorescence properties of a series of [n]cycloparaphenylene dications ([n]CPP2+, n = 5–9), which have unique in-plane aromaticity. The fluorescence peak positions of the [n]CPP2+s shifted to the longer-wavelength region with increasing ring size, reaching the near-infrared region for those with n > 5. The fluorescence quantum yield of [6]CPP2+ was the highest among the [n]CPP2+s examined in this study, and the value was on the same order as that of carbon nanotubes. The Stokes shifts of [n]CPP2+s were smaller than those of neutral [n]CPPs, which do not have in-plane aromaticity. Theoretical calculations indicate that [n]CPP2+s undergo smaller structural changes upon S0–S1 transition than [n]CPPs do, and this is responsible for the difference of the Stokes shift. Furthermore, molecular orbital analysis reveals that the S0–S1 transition of smaller [n]CPP2+s has an electric-dipole-forbidden character due to HOMO → LUMO/HOMO → LUMO+1 mixing. The relatively high fluorescence quantum yield of [6]CPP2+ is considered to arise from the balance between relatively allowed character and the dominant effect of energy gap.
中文翻译:

平面内芳香环对亚苯基阳离子的近红外荧光
环对亚苯基(CPP)是与富勒烯或扶手椅碳纳米管的部分结构相对应的箍状共轭烃。在这里,我们检查了具有独特的面内芳香性的一系列[ n ]环对亚苯基指示剂([ n ] CPP 2 +,n = 5–9)的荧光性质。[ n ] CPP 2+ s的荧光峰位置随着环尺寸的增加而移至较长波长区域,对于n > 5的那些峰达到近红外区域。[6] CPP 2+的荧光量子产率为[ n ] CPP 2+中最高在这项研究中进行了测试,其值与碳纳米管的数量级相同。[ n ] CPP 2+ s的斯托克斯位移小于不具有面内芳香性的中性[ n ] CPPs的斯托克斯位移。理论计算表明,[ n ] CPP 2+ s在S 0 –S 1跃迁时比[ n ] CPPs经历较小的结构变化,这是斯托克斯位移的差异所在。此外,分子轨道分析表明,较小的[ n ] CPP 2+的S 0 -S 1跃迁由于HOMO→LUMO / HOMO→LUMO + 1混合,因此s具有禁止电偶极的特性。认为[6] CPP 2+的相对较高的荧光量子产率是由于相对允许的特性与能隙的主导效应之间的平衡引起的。
更新日期:2018-05-18
中文翻译:

平面内芳香环对亚苯基阳离子的近红外荧光
环对亚苯基(CPP)是与富勒烯或扶手椅碳纳米管的部分结构相对应的箍状共轭烃。在这里,我们检查了具有独特的面内芳香性的一系列[ n ]环对亚苯基指示剂([ n ] CPP 2 +,n = 5–9)的荧光性质。[ n ] CPP 2+ s的荧光峰位置随着环尺寸的增加而移至较长波长区域,对于n > 5的那些峰达到近红外区域。[6] CPP 2+的荧光量子产率为[ n ] CPP 2+中最高在这项研究中进行了测试,其值与碳纳米管的数量级相同。[ n ] CPP 2+ s的斯托克斯位移小于不具有面内芳香性的中性[ n ] CPPs的斯托克斯位移。理论计算表明,[ n ] CPP 2+ s在S 0 –S 1跃迁时比[ n ] CPPs经历较小的结构变化,这是斯托克斯位移的差异所在。此外,分子轨道分析表明,较小的[ n ] CPP 2+的S 0 -S 1跃迁由于HOMO→LUMO / HOMO→LUMO + 1混合,因此s具有禁止电偶极的特性。认为[6] CPP 2+的相对较高的荧光量子产率是由于相对允许的特性与能隙的主导效应之间的平衡引起的。











































 京公网安备 11010802027423号
京公网安备 11010802027423号