European Journal of Medicinal Chemistry ( IF 6.0 ) Pub Date : 2018-05-18 , DOI: 10.1016/j.ejmech.2018.05.025 Yong Ling , Qiu-Xing Yang , Yu-Ning Teng , Shi Chen , Wei-Jie Gao , Jing Guo , Pei-Ling Hsu , Yue Liu , Susan L. Morris-Natschke , Chin-Chuan Hung , Kuo-Hsiung Lee
|
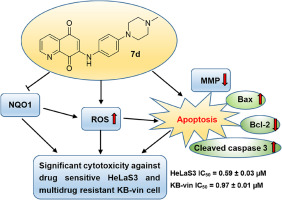
Fourteen novel amino-quinoline-5,8-dione derivatives (6a-h and 7a-h) were designed and synthesized by coupling different alkyl- or aryl-amino fragments at the C6- or C7-position of quinoline-5,8-dione. All target compounds showed antiproliferative potency in the low micromolar range in both drug sensitive HeLaS3 and multidrug resistant KB-vin cell lines. Compounds 6h, 6d, 7a, and 7d exhibited more potent antiproliferative effects than the other compounds. Especially, compounds 6d and 7d displayed NQO1-dependent cytotoxicity and competitive NQO1 inhibitory effects in both drug sensitive HeLaS3 and multidrug resistant KB-vin cell lines. Furthermore, compounds 6h, 6d, 7a, and 7d induced a dose-dependent lethal mitochondrial dysfunction in both drug sensitive HeLaS3 and multidrug resistant KB-vin cells by increasing intracellular reactive oxygen species (ROS) levels. Notably, compound 7d selectively inhibited cancer cells, but not non-tumor liver cell proliferation in vitro, and significantly triggered HeLaS3 cell apoptosis by regulating apoptotic proteins of Bcl-2, Bax, and cleaved caspase-3 in a dose-dependent manner. Our findings suggest that these novel C6- or C7-substituted amino-quinoline-5,8-dione derivatives, such as 7d, could be further developed in the future as potent and selective antitumor agents to potentially circumvent multi-drug resistance (MDR).
中文翻译:

新的氨基喹啉-5,8-二酮衍生物作为具有有效抗增殖活性的NAD(P)H:醌氧化还原酶1(NQO1)抑制剂的开发
通过在喹啉-5,8-的C6-或C7-位偶联不同的烷基或芳基氨基片段,设计并合成了十四种新颖的氨基喹啉-5,8-二酮衍生物(6a-h和7a-h)。 dione。在药物敏感性HeLaS3和耐多药KB-vin细胞系中,所有目标化合物均在低微摩尔范围内显示出抗增殖能力。与其他化合物相比,化合物6h,6d,7a和7d表现出更强的抗增殖作用。特别是化合物6d和7d在对药物敏感的HeLaS3和对多药耐药的KB-vin细胞系中均显示出NQO1依赖性的细胞毒性和竞争性NQO1抑制作用。此外,化合物6h,6d,7a和7d通过增加细胞内活性氧(ROS)水平,在药物敏感性HeLaS3和多药耐药KB-vin细胞中诱导了剂量依赖性致死线粒体功能障碍。值得注意的是,化合物7d在体外选择性抑制癌细胞,但不抑制非肿瘤肝细胞增殖并通过剂量依赖性方式调节Bcl-2,Bax和裂解的caspase-3凋亡蛋白显着触发HeLaS3细胞凋亡。我们的发现表明,这些新颖的C6或C7取代的氨基喹啉5,8-二酮衍生物(例如7d)将来可能会进一步开发,作为有效和选择性的抗肿瘤药物,可以潜在地规避多药耐药性(MDR) 。











































 京公网安备 11010802027423号
京公网安备 11010802027423号