Tetrahedron ( IF 2.1 ) Pub Date : 2018-05-18 , DOI: 10.1016/j.tet.2018.05.040 Kota Miyata , Satoru Yasuda , Takuto Masuya , Satoshi Ito , Yusuke Kinoshita , Hitoshi Tamiaki , Toru Oba
|
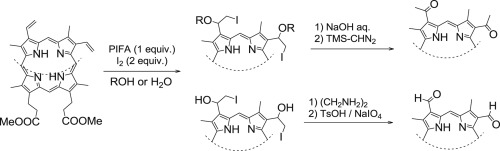
Iodination of protoporphyrin IX dimethyl ester using phenyliodine bis(trifluoroacetate) (PIFA) and I2 was studied. Iodine added to both the C3- and C8-vinyl groups equally to afford the iodohydrin or iodoether in the presence of water or alcohol, respectively. Any meso-hydrogen atom was not substituted by an iodine atom under these conditions, although both the vinyl group and one of the meso positions of methyl pyropheophorbide-a bearing a chlorin π-system, a chlorophyll-a derivative, was modified with PIFA and I2. The reaction intermediates derived from the porphyrin were more reactive than those from the chlorin and liable to form intermolecular linkages. The obtained 2-iodo-1-hydroxyethyl group was transformed into a formyl group by a mild treatment. The corresponding iodoether moiety was readily converted into the acetyl group under basic conditions. These transformations were also applicable to smaller olefins such as styrene.
中文翻译:

原卟啉IX二甲基酯中乙烯基的轻松碘化和碘化部分的后续转化
研究了使用苯基碘双(三氟乙酸盐)(PIFA)和I 2对原卟啉IX二甲酯进行碘化。碘同等地加到C3-和C8-乙烯基上,分别在水或醇存在下提供碘醇或碘醚。任何内消旋α-氢原子没有被在这些条件下,碘原子取代的,尽管这两个乙烯基和一个内消旋甲基的pyropheophorbide-位置一个轴承二氢卟酚的π系统,叶绿素一个衍生物,用PIFA和改性我2。来源于卟啉的反应中间体比来自二氢卟酚的反应中间体反应性更高,并且易于形成分子间键。通过温和的处理,将获得的2-碘-1-羟乙基转化为甲酰基。在碱性条件下,相应的碘醚部分易于转化为乙酰基。这些转变也适用于较小的烯烃,例如苯乙烯。











































 京公网安备 11010802027423号
京公网安备 11010802027423号