当前位置:
X-MOL 学术
›
Angew. Chem. Int. Ed.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
A Dimer of Hydrogen Cyanide Stabilized by a Lewis Acid
Angewandte Chemie International Edition ( IF 16.1 ) Pub Date : 2018-06-19 , DOI: 10.1002/anie.201804193 Kevin Bläsing 1 , Jonas Bresien 1 , René Labbow 1 , Axel Schulz 1, 2 , Alexander Villinger 1
Angewandte Chemie International Edition ( IF 16.1 ) Pub Date : 2018-06-19 , DOI: 10.1002/anie.201804193 Kevin Bläsing 1 , Jonas Bresien 1 , René Labbow 1 , Axel Schulz 1, 2 , Alexander Villinger 1
Affiliation
|
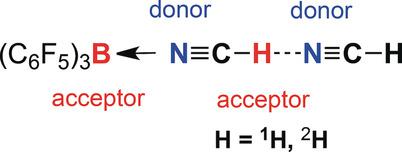
A highly labile dimer of hydrogen cyanide, HCN⋅⋅⋅HCN, was extracted from liquid HCN by adduct formation with the bulky Lewis acid B(C6F5)3, affording HCN⋅⋅⋅HCN−B(C6F5)3, which was fully characterized. The influence of the solvent (HCN, CH2Cl2, and aromatic hydrocarbons) on the crystallization process was studied, revealing dimer formation when using HCN or CH2Cl2 as solvent, whereas aromatic hydrocarbons led to the formation of monomeric arene⋅⋅HCN−B(C6F5)3 adducts, additionally stabilized by η6‐coordination of the aromatic ring system similar to well‐known half‐sandwich complexes.
中文翻译:

路易斯酸稳定的氰化氢二聚体
通过与笨重的路易斯酸B(C 6 F 5)3形成加合物,从液态HCN中提取出高度不稳定的氰化氢HCN····HCN二聚体,得到HCN···HCN -B(C 6 F 5)3,这是充分表征。研究了溶剂(HCN,CH 2 Cl 2和芳香烃)对结晶过程的影响,揭示了使用HCN或CH 2 Cl 2作为溶剂时二聚体的形成,而芳香烃导致单体芳烃的形成。 HCN-B(C 6 F 5)3加合物,另外由η稳定芳香环系统的6配位类似于众所周知的半三明治复合物。
更新日期:2018-06-19
中文翻译:

路易斯酸稳定的氰化氢二聚体
通过与笨重的路易斯酸B(C 6 F 5)3形成加合物,从液态HCN中提取出高度不稳定的氰化氢HCN····HCN二聚体,得到HCN···HCN -B(C 6 F 5)3,这是充分表征。研究了溶剂(HCN,CH 2 Cl 2和芳香烃)对结晶过程的影响,揭示了使用HCN或CH 2 Cl 2作为溶剂时二聚体的形成,而芳香烃导致单体芳烃的形成。 HCN-B(C 6 F 5)3加合物,另外由η稳定芳香环系统的6配位类似于众所周知的半三明治复合物。











































 京公网安备 11010802027423号
京公网安备 11010802027423号