当前位置:
X-MOL 学术
›
J. Phys. Chem. A
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
A Theoretical Study of Pyrolysis of exo-Tetrahydrodicyclopentadiene and Its Primary and Secondary Unimolecular Decomposition Products
The Journal of Physical Chemistry A ( IF 2.7 ) Pub Date : 2018-05-17 00:00:00 , DOI: 10.1021/acs.jpca.8b02934 Alexander N. Morozov 1 , Alexander M. Mebel 1 , Ralf I. Kaiser 2
The Journal of Physical Chemistry A ( IF 2.7 ) Pub Date : 2018-05-17 00:00:00 , DOI: 10.1021/acs.jpca.8b02934 Alexander N. Morozov 1 , Alexander M. Mebel 1 , Ralf I. Kaiser 2
Affiliation
|
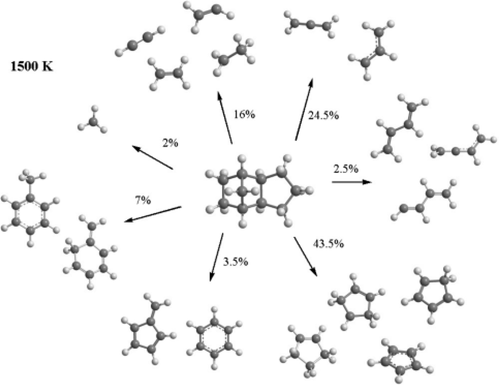
Theoretical calculations of the rate constants and product branching ratios in the pyrolysis of exo-tetrahydrodicyclopentadiene (JP-10) and its initial decomposition products at combustion-relevant pressures and temperatures were performed and compared to the experimental results from the recently reported molecular beam photoionization mass spectrometry study of the pyrolysis of JP-10 (Zhao et al. Phys. Chem. Chem. Phys. 2017, 19, 15780−15807). The results allow us to quantitatively assess the decomposition mechanisms of JP-10 by a direct comparison with the nascent product distribution—including radicals and thermally labile closed-shell species—detected in the short-residence-time molecular beam photoionization mass spectrometry experiment. In accord with the experimental data, the major products predicted by the theoretical modeling include methyl radical (CH3), acetylene (C2H2), vinyl radical (C2H3), ethyl radical (C2H5), ethylene (C2H4), allyl radical (C3H5), 1,3-butadiene (C4H6), cyclopentadienyl radical (C5H5), cyclopentadiene (C5H6), cyclopentenyl radical (C5H7), cyclopentene (C5H8), fulvene (C6H6), benzene (C6H6), toluene (C7H8), and 5-methylene-1,3-cyclohexadiene (C7H8). We found that ethylene, allyl radical, cyclopentadiene, and cyclopentenyl radical are significant products at all combustion-relevant conditions, whereas the relative yields of the other products depend on temperature. The most significant temperature trends predicted are increasing yields of the C2 and C4 species and decreasing yields of the C1, C6, and C7 groups with increasing temperature. The calculated pressure effect on the rate constant for the decomposition of JP-10 via initial C–H bond cleavages becomes significant at temperatures above 1500 K. The initially produced radicals will react away to form closed-shell molecules, such as ethylene, propene, cyclopentadiene, cyclopentene, fulvene, and benzene, which were observed as the predominant fragments in the long-residence-time experiment. The calculated rate constants and product branching ratios should prove useful to improve the existing kinetic models for the JP-10 pyrolysis.
中文翻译:

外四氢二环戊二烯及其一级和二级单分子分解产物热解的理论研究
理论计算了在燃烧相关的压力和温度下,exo -tetrahydrodicyclopentadiene(JP-10)及其初始分解产物在热解过程中的速率常数和产物支化比,并与最近报道的分子束光电离质量的实验结果进行了比较。 JP-10(Zhao等人的热分解的光谱研究的PHY。化学式化学物理。 2017年,19,15780−15807)。这些结果使我们能够通过与在短驻留时间分子束光电离质谱实验中检测到的新生产物分布(包括自由基和热不稳定的闭壳物种)进行直接比较,来定量评估JP-10的分解机理。根据实验数据,通过理论模型预测的主要产物包括甲基(CH 3),乙炔(C 2 H 2),乙烯基(C 2 H 3),乙基(C 2 H 5),乙烯(C 2 H 4),烯丙基(C 3 H 5),1,3-丁二烯(C 4 H 6),环戊二烯基(C 5 H 5),环戊二烯(C 5 H 6),环戊烯基(C 5 H 7),环戊烯(C 5 H 8),富烯( C 6 H 6),苯(C 6 H 6),甲苯(C 7 H 8)和5-亚甲基-1,3-环己二烯(C 7 H 8)。我们发现,在所有与燃烧相关的条件下,乙烯,烯丙基,环戊二烯和环戊烯基都是重要的产物,而其他产物的相对产率则取决于温度。预测的最显着的温度趋势是C2和C4物种的产量增加,而C1,C6和C7组的产量随着温度的升高而降低。在高于1500 K的温度下,计算得出的压力对JP-10通过初始C–H键断裂的分解速率常数的影响变得显着。最初产生的自由基会发生反应,形成闭壳分子,例如乙烯,丙烯,在长时间实验中,环戊二烯,环戊烯,富勒烯和苯是主要的碎片。
更新日期:2018-05-17
中文翻译:

外四氢二环戊二烯及其一级和二级单分子分解产物热解的理论研究
理论计算了在燃烧相关的压力和温度下,exo -tetrahydrodicyclopentadiene(JP-10)及其初始分解产物在热解过程中的速率常数和产物支化比,并与最近报道的分子束光电离质量的实验结果进行了比较。 JP-10(Zhao等人的热分解的光谱研究的PHY。化学式化学物理。 2017年,19,15780−15807)。这些结果使我们能够通过与在短驻留时间分子束光电离质谱实验中检测到的新生产物分布(包括自由基和热不稳定的闭壳物种)进行直接比较,来定量评估JP-10的分解机理。根据实验数据,通过理论模型预测的主要产物包括甲基(CH 3),乙炔(C 2 H 2),乙烯基(C 2 H 3),乙基(C 2 H 5),乙烯(C 2 H 4),烯丙基(C 3 H 5),1,3-丁二烯(C 4 H 6),环戊二烯基(C 5 H 5),环戊二烯(C 5 H 6),环戊烯基(C 5 H 7),环戊烯(C 5 H 8),富烯( C 6 H 6),苯(C 6 H 6),甲苯(C 7 H 8)和5-亚甲基-1,3-环己二烯(C 7 H 8)。我们发现,在所有与燃烧相关的条件下,乙烯,烯丙基,环戊二烯和环戊烯基都是重要的产物,而其他产物的相对产率则取决于温度。预测的最显着的温度趋势是C2和C4物种的产量增加,而C1,C6和C7组的产量随着温度的升高而降低。在高于1500 K的温度下,计算得出的压力对JP-10通过初始C–H键断裂的分解速率常数的影响变得显着。最初产生的自由基会发生反应,形成闭壳分子,例如乙烯,丙烯,在长时间实验中,环戊二烯,环戊烯,富勒烯和苯是主要的碎片。









































 京公网安备 11010802027423号
京公网安备 11010802027423号