当前位置:
X-MOL 学术
›
J. Phys. Chem. A
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Spectroscopy of Proton Coordination with Ethylenediamine
The Journal of Physical Chemistry A ( IF 2.9 ) Pub Date : 2018-05-17 00:00:00 , DOI: 10.1021/acs.jpca.8b03592 J. Philipp Wagner 1 , David C. McDonald 1 , Michael A. Duncan 1
The Journal of Physical Chemistry A ( IF 2.9 ) Pub Date : 2018-05-17 00:00:00 , DOI: 10.1021/acs.jpca.8b03592 J. Philipp Wagner 1 , David C. McDonald 1 , Michael A. Duncan 1
Affiliation
|
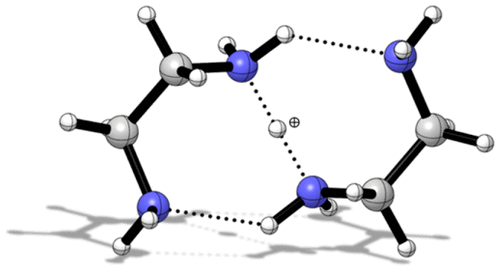
Protonated ethylenediamine monomer, dimer, and trimer were produced in the gas phase by an electrical discharge/supersonic expansion of argon seeded with ethylenediamine (C2H8N2, en) vapor. Infrared spectra of these ions were measured in the region from 1000 to 4000 cm–1 using laser photodissociation and argon tagging. Computations at the CBS-QB3 level were performed to explore possible isomers and understand the infrared spectra. The protonated monomer exhibits a gauche conformation and an intramolecular hydrogen bond. Its parallel shared proton vibration occurs as a broad band around 2785 cm–1, despite the formally equivalent proton affinities of the two amino groups involved, which usually leads to low frequency bands. The barrier to intramolecular proton transfer is 2.2 kcal mol–1 and does not vanish upon addition of the zero-point energy, unlike the related protonated ammonia dimer. The structure of the dimer is formed by chelation of the monomer’s NH3+ group, thereby localizing the excess proton and increasing the frequency of the intramolecular shared proton vibration to 3157 cm–1. Other highly fluxional dimer structures with facile intermolecular proton transfer and concomitant structural reorganization were computed to lie within 2 kcal mol–1 of the experimentally observed structure. The spectrum of the trimer is rather diffuse, and a clear assignment is not possible. However, an isomer with an intramolecular proton transfer like that of the monomer is most consistent with the experimental spectrum.
中文翻译:

乙二胺与质子配位的光谱学
质子化的乙二胺单体,二聚体和三聚体在气相中通过乙二胺(C 2 H 8 N 2,en)蒸气注入的氩气的放电/超音速膨胀产生。使用激光光解和氩标记,在1000至4000 cm –1范围内测量了这些离子的红外光谱。进行了CBS-QB3一级的计算,以探索可能的异构体并了解红外光谱。质子化的单体表现出gauche构象和分子内氢键。其平行的共享质子振动发生在2785 cm –1左右的宽带处,尽管所涉及的两个氨基在形式上等同于质子亲和力,通常会导致低频带。与相关的质子化氨二聚体不同,分子内质子转移的障碍为2.2 kcal mol –1,并且在添加零点能量时不会消失。二聚体的结构是通过单体的NH 3 +基团的螯合形成的,从而使过量的质子局部化,并使分子内共享质子振动的频率增加到3157 cm –1。计算出的其他具有分子间质子转移和伴随结构重组的高通量二聚体结构位于2 kcal mol –1之内实验观察到的结构 三聚体的光谱相当分散,不可能进行明确的分配。但是,具有分子内质子转移的异构体(如单体的异构体)与实验光谱最一致。
更新日期:2018-05-17
中文翻译:

乙二胺与质子配位的光谱学
质子化的乙二胺单体,二聚体和三聚体在气相中通过乙二胺(C 2 H 8 N 2,en)蒸气注入的氩气的放电/超音速膨胀产生。使用激光光解和氩标记,在1000至4000 cm –1范围内测量了这些离子的红外光谱。进行了CBS-QB3一级的计算,以探索可能的异构体并了解红外光谱。质子化的单体表现出gauche构象和分子内氢键。其平行的共享质子振动发生在2785 cm –1左右的宽带处,尽管所涉及的两个氨基在形式上等同于质子亲和力,通常会导致低频带。与相关的质子化氨二聚体不同,分子内质子转移的障碍为2.2 kcal mol –1,并且在添加零点能量时不会消失。二聚体的结构是通过单体的NH 3 +基团的螯合形成的,从而使过量的质子局部化,并使分子内共享质子振动的频率增加到3157 cm –1。计算出的其他具有分子间质子转移和伴随结构重组的高通量二聚体结构位于2 kcal mol –1之内实验观察到的结构 三聚体的光谱相当分散,不可能进行明确的分配。但是,具有分子内质子转移的异构体(如单体的异构体)与实验光谱最一致。



























 京公网安备 11010802027423号
京公网安备 11010802027423号