Journal of Inorganic Biochemistry ( IF 3.8 ) Pub Date : 2018-05-18 , DOI: 10.1016/j.jinorgbio.2018.05.008 David S. Weber , Jeffrey J. Warren
|
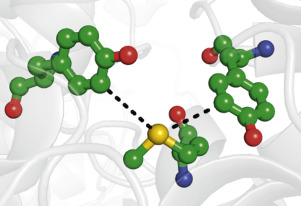
Redox reactions of the aromatic amino acids tyrosine (Tyr) and tryptophan (Trp) are crucial for the biological functions of many metalloproteins. An important question is how biological systems can use the protein environment to move electrons through proteins in a controlled manner. Methionine (Met)-aromatic interactions are common in proteins, but little is known about redox reactions of such motifs. Here, we explore methionine sulfur-aromatic interactions in the oxidoreductase (EC 1) class of proteins and their proximity to metal sites. We also propose a new metric for classifying Met-aromatic interactions called “interaction order.” Over 12,000 protein structures from the Protein Data Bank were analyzed. A linear algebraic heuristic was used to classify the interaction of Met‑sulfur with tyrosine, tryptophan, and phenylalanine. We found that 83% of oxidoreductase proteins contained aromatic interactions meeting our criteria, with a preferential angle of about 60° between Met‑sulfur lone pairs and aromatic planes. A total of 41% of Met-aromatic interactions meeting our criteria were found to be within 20 Å of a metal site, and 6% were found within 10 Å. A surprising number of “bridging” interactions, involving two aromatic residues and one Met also were identified. Finally, selected examples of potentially important Met-aromatic redox motifs are outlined. On the basis of our results, we suggest that Met-aromatic interactions should be considered as mediators of electron transfer reactions, as well as their more widely recognized roles as structural motifs.
中文翻译:

在酶的氧化还原酶类别中对蛋氨酸-芳香族相互作用的几何结构进行的调查:在金属位点附近,Met-芳香族相互作用会发生什么?
芳香族氨基酸酪氨酸(Tyr)和色氨酸(Trp)的氧化还原反应对于许多金属蛋白的生物学功能至关重要。一个重要的问题是生物系统如何利用蛋白质环境以受控方式使电子通过蛋白质。蛋氨酸(Met)-芳香族相互作用在蛋白质中很常见,但对于此类基序的氧化还原反应知之甚少。在这里,我们探讨蛋白质的氧化还原酶(EC 1)类中的蛋氨酸硫-芳香族相互作用及其与金属位点的接近性。我们还提出了一种新的衡量Met-芳香族相互作用的指标,称为“相互作用顺序”。分析了来自蛋白质数据库的12,000多种蛋白质结构。线性代数启发法用于分类Met-硫与酪氨酸,色氨酸和苯丙氨酸的相互作用。我们发现83%的氧化还原酶蛋白包含符合我们标准的芳香族相互作用,在Met-硫磺孤对与芳香族平面之间存在约60°的优先角。满足我们标准的Met-芳香族相互作用中,总共有41%被发现在金属位点20Å之内,而在10Å内发现了6%。还发现了惊人数量的“桥连”相互作用,其中涉及两个芳族残基和一个大都会。最后,概述了潜在重要的Met-芳香族氧化还原基序的选定示例。根据我们的结果,我们建议应将Met-芳香族相互作用视为电子转移反应的介质,并将其作为结构基序的作用得到广泛认可。在Met-硫孤对与芳香族平面之间的优先角约为60°。满足我们标准的Met-芳香族相互作用中,总共有41%被发现在金属位点20Å之内,而在10Å内发现了6%。还发现了惊人数量的“桥连”相互作用,其中涉及两个芳族残基和一个大都会。最后,概述了潜在重要的Met-芳香族氧化还原基序的选定示例。根据我们的结果,我们建议应将Met-芳香族相互作用视为电子转移反应的介质,并将其作为结构基序的作用得到广泛认可。在Met-硫孤对与芳香族平面之间的优先角约为60°。满足我们标准的Met-芳香族相互作用中,总共有41%被发现在金属位点20Å之内,而在10Å内发现了6%。还发现了惊人数量的“桥连”相互作用,其中涉及两个芳族残基和一个大都会。最后,概述了潜在重要的Met-芳香族氧化还原基序的选定示例。根据我们的结果,我们建议应将Met-芳香族相互作用视为电子转移反应的介质,并将其作为结构基序的作用得到广泛认可。还发现了惊人数量的“桥连”相互作用,其中涉及两个芳族残基和一个大都会。最后,概述了潜在重要的Met-芳香族氧化还原基序的选定示例。根据我们的结果,我们建议应将Met-芳香族相互作用视为电子转移反应的介质,并将其作为结构基序的作用得到广泛认可。还发现了惊人数量的“桥连”相互作用,其中涉及两个芳族残基和一个大都会。最后,概述了潜在重要的Met-芳香族氧化还原基序的选定示例。根据我们的结果,我们建议应将Met-芳香族相互作用视为电子转移反应的介质,并将其作为结构基序的作用得到广泛认可。











































 京公网安备 11010802027423号
京公网安备 11010802027423号