European Journal of Medicinal Chemistry ( IF 6.0 ) Pub Date : 2018-05-18 , DOI: 10.1016/j.ejmech.2018.05.018 Nicolo Tonali , Julia Kaffy , Jean-Louis Soulier , Maria Luisa Gelmi , Emanuela Erba , Myriam Taverna , Carine van Heijenoort , Tap Ha-Duong , Sandrine Ongeri
|
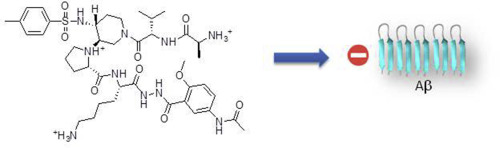
Aggregation of amyloid proteins is currently involved in more than 20 serious human diseases that are actually untreated, such as Alzheimer's disease (AD). Despite many efforts made to target the amyloid cascade in AD, finding an aggregation inhibiting compound and especially modulating early oligomerization remains a relevant and challenging strategy. We report herein the first examples of small and non-peptide mimics of acyclic beta-hairpins, showing an ability to delay the fibrillization of amyloid-β (Aβ1-42) peptide and deeply modify its early oligomerization process. Modifications providing better druggability properties such as increased hydrophilicity and reduced peptidic character were performed. We also demonstrate that an appropriate balance between flexibility and stability of the β-hairpin must be reached to adapt to the different shape of the various aggregated forms of the amyloid peptide. This strategy can be investigated to target other challenging amyloid proteins.
中文翻译:

β-发夹模拟物作为淀粉样β-肽聚集调节剂的结构-活性关系
淀粉样蛋白的聚集目前涉及实际上未得到治疗的20多种严重的人类疾病,例如阿尔茨海默氏病(AD)。尽管为靶向AD中的淀粉样蛋白级联做出了许多努力,但是发现抑制聚集的化合物,尤其是调节早期低聚仍然是一个相关且具有挑战性的策略。我们在此报告无环β-发夹结构的小肽和非肽模拟物的第一个实例,显示了延迟淀粉样蛋白-β(Aβ1-42)肽,并深刻修饰其早期的低聚过程。进行了提供更好的可药用性(例如增加的亲水性和降低的肽特性)的修饰。我们还证明,必须适应β-发夹的柔性和稳定性之间的适当平衡,以适应淀粉样肽的各种聚集形式的不同形状。可以研究此策略以靶向其他具有挑战性的淀粉样蛋白。











































 京公网安备 11010802027423号
京公网安备 11010802027423号