当前位置:
X-MOL 学术
›
Environ. Sci. Technol.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Oxidation of Cr(III)-Fe(III) Mixed-Phase Hydroxides by Chlorine: Implications on the Control of Hexavalent Chromium in Drinking Water.
Environmental Science & Technology ( IF 10.8 ) Pub Date : 2018-07-06 , DOI: 10.1021/acs.est.7b06013 Michelle Chebeir 1 , Haizhou Liu 1
Environmental Science & Technology ( IF 10.8 ) Pub Date : 2018-07-06 , DOI: 10.1021/acs.est.7b06013 Michelle Chebeir 1 , Haizhou Liu 1
Affiliation
|
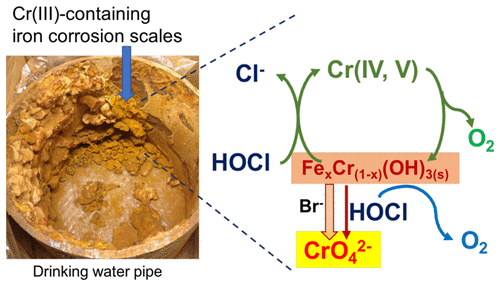
The occurrence of chromium (Cr) as an inorganic contaminant in drinking water is widely reported. One source of Cr is its accumulation in iron-containing corrosion scales of drinking water distribution systems as Cr(III)-Fe(III) hydroxide, that is, Fe xCr(1- x)(OH)3(s), where x represents the Fe(III) molar content and typically varies between 0.25 and 0.75. This study investigated the kinetics of inadvertent hexavalent chromium Cr(VI) formation via the oxidation of Fe xCr(1- x)(OH)3(s) by chlorine as a residual disinfectant in drinking water, and examined the impacts of Fe(III) content and drinking water chemical parameters including pH, bromide and bicarbonate on the rate of Cr(VI) formation. Data showed that an increase in Fe(III) molar content resulted in a significant decrease in the stoichiometric Cr(VI) yield and the rate of Cr(VI) formation, mainly due to chlorine decay induced by Fe(III) surface sites. An increase in bicarbonate enhanced the rate of Cr(VI) formation, likely due to the formation of Fe(III)-carbonato surface complexes that slowed down the scavenging reaction with chlorine. The presence of bromide significantly accelerated the oxidation of Fe xCr(1- x)(OH)3(s) by chlorine, resulting from the catalytic effect of bromide acting as an electron shuttle. A higher solution pH between 6 and 8.5 slowed down the oxidation of Cr(III) by chlorine. These findings suggested that the oxidative conversion of chromium-containing iron corrosion products in drinking water distribution systems can lead to the occurrence of Cr(VI) at the tap, and the abundance of iron, and a careful control of pH, bicarbonate and bromide levels can assist the control of Cr(VI) formation.
中文翻译:

Cr氧化Cr(III)-Fe(III)混合相氢氧化物:对控制饮用水中六价铬的影响。
饮用水中无机污染物铬(Cr)的存在被广泛报道。Cr的一种来源是其在饮用水分配系统的含铁腐蚀垢中以Cr(III)-Fe(III)氢氧化物的形式积累,即Fe xCr(1- x)(OH)3(s),其中x表示Fe(III)的摩尔含量,通常在0.25到0.75之间变化。这项研究调查了作为饮用水中残留消毒剂的氯对Fe xCr(1- x)(OH)3(s)的氧化而无意中形成的六价铬Cr(VI)的动力学,并研究了Fe(III)的影响。含量和饮用水中的化学参数,包括pH,溴化物和碳酸氢根对Cr(VI)生成速率的影响。数据表明,Fe(III)摩尔含量的增加导致化学计量的Cr(VI)产率和Cr(VI)形成速率显着降低,主要是由于Fe(III)表面位点引起的氯气衰减。碳酸氢盐的增加提高了Cr(VI)的形成速率,这可能是由于Fe(III)-碳酸盐表面配合物的形成减慢了与氯的清除反应。溴化物的存在显着促进了Fe xCr(1- x)(OH)3(s)被氯氧化,这是由于溴化物起着电子穿梭的催化作用。较高的溶液pH在6和8.5之间,减缓了氯对Cr(III)的氧化。这些发现表明,饮用水分配系统中含铬铁腐蚀产物的氧化转化可导致水龙头中Cr(VI)的发生,铁的丰度以及对pH,碳酸氢盐和溴化物水平的谨慎控制。可以帮助控制Cr(VI)的形成。
更新日期:2018-07-08
中文翻译:

Cr氧化Cr(III)-Fe(III)混合相氢氧化物:对控制饮用水中六价铬的影响。
饮用水中无机污染物铬(Cr)的存在被广泛报道。Cr的一种来源是其在饮用水分配系统的含铁腐蚀垢中以Cr(III)-Fe(III)氢氧化物的形式积累,即Fe xCr(1- x)(OH)3(s),其中x表示Fe(III)的摩尔含量,通常在0.25到0.75之间变化。这项研究调查了作为饮用水中残留消毒剂的氯对Fe xCr(1- x)(OH)3(s)的氧化而无意中形成的六价铬Cr(VI)的动力学,并研究了Fe(III)的影响。含量和饮用水中的化学参数,包括pH,溴化物和碳酸氢根对Cr(VI)生成速率的影响。数据表明,Fe(III)摩尔含量的增加导致化学计量的Cr(VI)产率和Cr(VI)形成速率显着降低,主要是由于Fe(III)表面位点引起的氯气衰减。碳酸氢盐的增加提高了Cr(VI)的形成速率,这可能是由于Fe(III)-碳酸盐表面配合物的形成减慢了与氯的清除反应。溴化物的存在显着促进了Fe xCr(1- x)(OH)3(s)被氯氧化,这是由于溴化物起着电子穿梭的催化作用。较高的溶液pH在6和8.5之间,减缓了氯对Cr(III)的氧化。这些发现表明,饮用水分配系统中含铬铁腐蚀产物的氧化转化可导致水龙头中Cr(VI)的发生,铁的丰度以及对pH,碳酸氢盐和溴化物水平的谨慎控制。可以帮助控制Cr(VI)的形成。











































 京公网安备 11010802027423号
京公网安备 11010802027423号