Acta Biomaterialia ( IF 9.4 ) Pub Date : 2018-05-18 , DOI: 10.1016/j.actbio.2018.05.027 Agnese Carino , Christian Ludwig , Antonio Cervellino , Elisabeth Müller , Andrea Testino
|
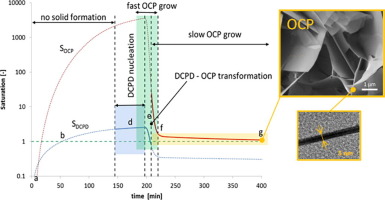
The experimental data on calcium phosphates formation were collected in dilute solution at constant pH (7.40) and temperature (37.0 °C) at different levels of ionic strength (IS). The evolution of the solid phase formation is described in detail using a thermodynamic-kinetic model. The thermodynamic model takes into account all relevant chemical species as well as Posner’s clusters; the kinetic model, based on the discretized population balance approach, accounts for the solid formation from solution. The experimental data are consistent with an initial formation of dicalcium phosphate dihydrate (DCPD, brushite), which dominates the nucleation rate, and its rapid transformation into octacalcium phosphate (OCP) or hydroxyapatite (HA), which dominates the growth rate. Depending on the experimental conditions and, including the influence of the IS level, OCP may be further transformed into apatite. The classical nucleation theory is able to describe the experimental results very well and the solid phase growth is limited by the diffusion of Ca2+ ions. The precipitation pathway described by a complete thermodynamic-kinetic model is expected to contribute to the understating of the in vivo osteogenesis.
Statement of significance
The formation mechanism of calcium phosphates under biomimetic conditions is unraveled. The formation pathway is mathematically described based on a thermodynamic-kinetic model in which (i) the nucleation stages (primary and secondary) are dominated by the formation of dicalcium phosphate dihydrate (DCPD) and (ii) the fast growth stage is limited by the diffusion of Ca2+ ions under the driving force of octacalcium phosphate (OCP), or hydroxyapatite (HA), solubility. The obtained solid phase seems correlated to the activity coefficient of phosphate ions, thus to the ionic strength and local phosphate speciation. The model, being able to highlight the details of the precipitation pathway, is expected to contribute to the understanding of the apatitic phase formation in the biomineralization-biodemineralization processes under in-vivo conditions.
中文翻译:

生物相关条件下磷酸钙相的形成和转化:实验和建模。
在恒定pH(7.40)和温度(37.0°C)下,在不同的离子强度(IS)水平下,在稀溶液中收集了有关磷酸钙形成的实验数据。使用热动力学模型详细描述了固相形成的演变。热力学模型考虑了所有相关的化学物种以及波斯纳团簇。基于离散种群平衡方法的动力学模型解释了溶液的固体形成。实验数据与最初形成二水合磷酸氢钙(DCPD,透钙磷石)相一致,后者主要控制成核速率,并迅速转变为主要控制生长速率的磷酸八钙(OCP)或羟基磷灰石(HA)。根据实验条件,包括IS水平的影响,OCP可能会进一步转变为磷灰石。经典的成核理论能够很好地描述实验结果,并且固相的生长受到Ca扩散的限制。2+离子。完整的热动力学模型描述的沉淀途径有望有助于体内成骨作用的低估。
重要声明
仿生条件下磷酸钙的形成机理尚未阐明。基于热力学模型对形成途径进行数学描述,其中(i)成核阶段(主要和次要)主要由二水合磷酸二钙(DCPD)的形成和(ii)快速生长阶段受到在磷酸八钙(OCP)或羟基磷灰石(HA)的驱动力下Ca2 +离子的扩散,溶解度。所获得的固相似乎与磷酸根离子的活性系数相关,因此与离子强度和局部磷酸根形态相关。该模型能够突出沉淀途径的细节,预计将有助于了解体内条件下生物矿化-生物脱矿质过程中的磷化相形成。











































 京公网安备 11010802027423号
京公网安备 11010802027423号