Bioorganic Chemistry ( IF 4.5 ) Pub Date : 2018-05-17 , DOI: 10.1016/j.bioorg.2018.05.012 María Julia Castro , Victoria Richmond , María Belén Faraoni , Ana Paula Murray
|
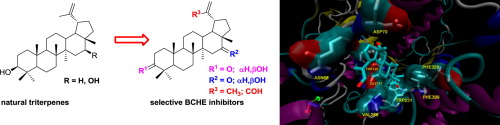
A set of triterpenoids with different grades of oxidation in the lupane skeleton were prepared and evaluated as cholinesterase inhibitors. Allylic oxidation with selenium oxide and Jones’s oxidation were employed to obtain mono-, di- and tri-oxolupanes, starting from calenduladiol (1) and lupeol (3). All the derivatives showed a selective inhibition of butyrylcholinesterase over acetylcholinesterase (BChE vs. AChE). A kinetic study proved that compounds 2 and 9, the more potent inhibitors of the series, act as competitive inhibitors. Molecular modeling was used to understand their interaction with BChE, the role of carbonyl at C-16 and the selectivity towards this enzyme over AChE. These results indicate that oxidation at C-16 of the lupane skeleton is a key transformation in order to improve the cholinesterase inhibition of these compounds.
中文翻译:

C-16处的氧化可增强三聚戊烷中丁酰胆碱酯酶的抑制作用
制备了一组在三聚戊二烯骨架中具有不同氧化程度的三萜类化合物,并将其作为胆碱酯酶抑制剂进行评估。从金盏花二醇(1)和羽扇豆酚(3)开始,用氧化硒进行烯丙基氧化和琼斯氧化来获得一,二和三氧杂双环戊烷。所有衍生物均显示出丁酰胆碱酯酶比乙酰胆碱酯酶的选择性抑制作用(BChE与AChE)。动力学研究证明化合物2和9,该系列中最有效的抑制剂,起竞争性抑制剂的作用。使用分子建模来了解它们与BChE的相互作用,羰基在C-16上的作用以及对该酶的选择性(相对于AChE)。这些结果表明,为了改善这些化合物对胆碱酯酶的抑制作用,在羽扇豆骨架的C-16处的氧化是关键的转化。











































 京公网安备 11010802027423号
京公网安备 11010802027423号