当前位置:
X-MOL 学术
›
ChemMedChem
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Cytotoxic Activity and Structure–Activity Relationship of Triazole‐Containing Bis(Aryl Ether) Macrocycles
ChemMedChem ( IF 3.6 ) Pub Date : 2018-05-17 , DOI: 10.1002/cmdc.201800075 Eduardo Hernández-Vázquez 1 , Alejandra Chávez-Riveros 1 , Adriana Romo-Pérez 1 , María Teresa Ramírez-Apán 1 , Alma D. Chávez-Blanco 2 , Rocío Morales-Bárcenas 2 , Alfonso Dueñas-González 3 , Luis D. Miranda 1
ChemMedChem ( IF 3.6 ) Pub Date : 2018-05-17 , DOI: 10.1002/cmdc.201800075 Eduardo Hernández-Vázquez 1 , Alejandra Chávez-Riveros 1 , Adriana Romo-Pérez 1 , María Teresa Ramírez-Apán 1 , Alma D. Chávez-Blanco 2 , Rocío Morales-Bárcenas 2 , Alfonso Dueñas-González 3 , Luis D. Miranda 1
Affiliation
|
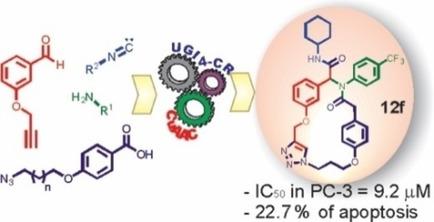
Cancer continues to be a worldwide health problem. Certain macrocyclic molecules have become attractive therapeutic alternatives for this disease because of their efficacy and, frequently, their novel mechanisms of action. Herein, we report the synthesis of a series of 20‐, 21‐, and 22‐membered macrocycles containing triazole and bis(aryl ether) moieties. The compounds were prepared by a multicomponent approach from readily available commercial substrates. Notably, some of the compounds displayed interesting cytotoxicity against cancer (PC‐3) and breast (MCF‐7) cell lines, especially those bearing an aliphatic or a trifluoromethyl substituent on the N‐phenyl moiety (IC50<13 μm). Additionally, some of the compounds were able to induce apoptosis relative to the solvent control; in particular, (Z)‐N‐cyclohexyl‐7‐oxo‐6‐[4‐(trifluoromethyl)phenyl]‐11H‐3,10‐dioxa‐6‐aza‐1(4,1)‐triazola‐4(1,3),9(1,4)‐dibenzenacyclotridecaphane‐5‐carboxamide (12 f) was the most potent in this regard (22.7 % of apoptosis).
中文翻译:

含三唑的双(芳醚)大环化合物的细胞毒活性和构效关系
癌症仍然是世界范围内的健康问题。某些大环分子由于其功效,并且经常是其新的作用机理,已成为该疾病的有吸引力的治疗选择。在这里,我们报告了一系列包含三唑和双(芳基醚)部分的20、21和22元大环的合成。通过多组分方法从容易获得的商业底物制备化合物。值得注意的是,一些化合物显示细胞毒性有趣针对癌症(PC-3)和乳腺癌(MCF-7)细胞系,尤其是那些带有脂族或在一个三氟甲基取代基ñ -苯基部分(IC 50 <13μ米)。另外,相对于溶剂对照,某些化合物能够诱导细胞凋亡。特别是(Z)-N-环己基-7-氧代-6- [4-(三氟甲基)苯基] -1 1 H-3,10-二氧杂-6-氮杂-1(4,1)-三唑-4 (1,3),9(1,4)-苯甲环上环己酮-5-羧酰胺(12 f)在这方面是最有效的(凋亡的22.7%)。
更新日期:2018-05-17
中文翻译:

含三唑的双(芳醚)大环化合物的细胞毒活性和构效关系
癌症仍然是世界范围内的健康问题。某些大环分子由于其功效,并且经常是其新的作用机理,已成为该疾病的有吸引力的治疗选择。在这里,我们报告了一系列包含三唑和双(芳基醚)部分的20、21和22元大环的合成。通过多组分方法从容易获得的商业底物制备化合物。值得注意的是,一些化合物显示细胞毒性有趣针对癌症(PC-3)和乳腺癌(MCF-7)细胞系,尤其是那些带有脂族或在一个三氟甲基取代基ñ -苯基部分(IC 50 <13μ米)。另外,相对于溶剂对照,某些化合物能够诱导细胞凋亡。特别是(Z)-N-环己基-7-氧代-6- [4-(三氟甲基)苯基] -1 1 H-3,10-二氧杂-6-氮杂-1(4,1)-三唑-4 (1,3),9(1,4)-苯甲环上环己酮-5-羧酰胺(12 f)在这方面是最有效的(凋亡的22.7%)。











































 京公网安备 11010802027423号
京公网安备 11010802027423号