当前位置:
X-MOL 学术
›
ChemMedChem
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Structural Analysis of Small‐Molecule Binding to the BAZ2A and BAZ2B Bromodomains
ChemMedChem ( IF 3.4 ) Pub Date : 2018-06-21 , DOI: 10.1002/cmdc.201800234 Andrea Dalle Vedove 1 , Dimitrios Spiliotopoulos 2 , Vito G. D'Agostino 1 , Jean-Rémy Marchand 2 , Andrea Unzue 3 , Cristina Nevado 3 , Graziano Lolli 1 , Amedeo Caflisch 2
ChemMedChem ( IF 3.4 ) Pub Date : 2018-06-21 , DOI: 10.1002/cmdc.201800234 Andrea Dalle Vedove 1 , Dimitrios Spiliotopoulos 2 , Vito G. D'Agostino 1 , Jean-Rémy Marchand 2 , Andrea Unzue 3 , Cristina Nevado 3 , Graziano Lolli 1 , Amedeo Caflisch 2
Affiliation
|
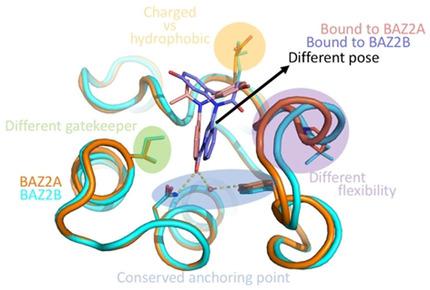
The bromodomain‐containing protein BAZ2A is a validated target in prostate cancer research, whereas the function of its paralogue BAZ2B is still undefined. The bromodomains of BAZ2A and BAZ2B have a similar binding site for their natural ligand, the acetylated lysine side chain. Here, we present an analysis of the binding modes of eight compounds belonging to three distinct chemical classes. For all compounds, the moiety mimicking the natural ligand engages in essentially identical interactions in the BAZ2A and BAZ2B bromodomains. In contrast, the rest of the molecule is partially solvent‐exposed and adopts different orientations with different interactions in the two bromodomains. Some of these differences could be exploited for designing inhibitors with selectivity within the BAZ2 bromodomain subfamily.
中文翻译:

小分子与BAZ2A和BAZ2B溴结构域结合的结构分析
含溴结构域的蛋白BAZ2A在前列腺癌研究中是经过验证的靶标,而其旁系蛋白BAZ2B的功能仍不确定。BAZ2A和BAZ2B的溴结构域对它们的天然配体乙酰化的赖氨酸侧链具有相似的结合位点。在这里,我们对属于三种不同化学类别的八种化合物的结合模式进行了分析。对于所有化合物,模拟天然配体的部分在BAZ2A和BAZ2B溴结构域中参与基本相同的相互作用。相比之下,其余分子则部分暴露于溶剂中,并且在两个溴结构域中采用不同的方向和不同的相互作用。这些差异中的一些可用于设计BAZ2溴结构域亚家族内的选择性抑制剂。
更新日期:2018-06-21
中文翻译:

小分子与BAZ2A和BAZ2B溴结构域结合的结构分析
含溴结构域的蛋白BAZ2A在前列腺癌研究中是经过验证的靶标,而其旁系蛋白BAZ2B的功能仍不确定。BAZ2A和BAZ2B的溴结构域对它们的天然配体乙酰化的赖氨酸侧链具有相似的结合位点。在这里,我们对属于三种不同化学类别的八种化合物的结合模式进行了分析。对于所有化合物,模拟天然配体的部分在BAZ2A和BAZ2B溴结构域中参与基本相同的相互作用。相比之下,其余分子则部分暴露于溶剂中,并且在两个溴结构域中采用不同的方向和不同的相互作用。这些差异中的一些可用于设计BAZ2溴结构域亚家族内的选择性抑制剂。



























 京公网安备 11010802027423号
京公网安备 11010802027423号