当前位置:
X-MOL 学术
›
Org. Chem. Front.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
The iridium-catalysed reductive coupling reaction of tertiary lactams/amides with isocyanoacetates†
Organic Chemistry Frontiers ( IF 4.6 ) Pub Date : 2018-05-17 00:00:00 , DOI: 10.1039/c8qo00312b Xiu-Ning Hu 1, 2, 3, 4, 5 , Tai-Long Shen 1, 2, 3, 4, 5 , Dong-Cheng Cai 1, 2, 3, 4, 5 , Jian-Feng Zheng 1, 2, 3, 4, 5 , Pei-Qiang Huang 1, 2, 3, 4, 5
Organic Chemistry Frontiers ( IF 4.6 ) Pub Date : 2018-05-17 00:00:00 , DOI: 10.1039/c8qo00312b Xiu-Ning Hu 1, 2, 3, 4, 5 , Tai-Long Shen 1, 2, 3, 4, 5 , Dong-Cheng Cai 1, 2, 3, 4, 5 , Jian-Feng Zheng 1, 2, 3, 4, 5 , Pei-Qiang Huang 1, 2, 3, 4, 5
Affiliation
|
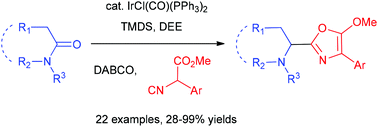
Catalytic C–C bond forming methods for the one-pot transformation of amides into other classes of compounds are highly demanding. In this report, we demonstrate the catalytic reductive addition of isocyanoacetates to tertiary lactams/amides to produce 5-methoxyoxazoles, including N-heterocyclic substituted oxazoles and sterically hindered amino oxazoles that are difficult to obtain by traditional oxazole-forming Ugi-type reactions. This one-pot procedure involves the Ir-catalysed partial reduction of lactams/amides and the sequential chemoselective addition of the isocyanide group in isocyanoacetates.
中文翻译:

铱催化的内酰胺/酰胺与异氰基乙酸盐的还原偶联反应†
对于将酰胺一锅转化为其他类型化合物的催化CC键形成方法提出了很高的要求。在此报告中,我们证明了将异氰基乙酸酯催化还原加成到叔内酰胺/酰胺上可生成5-甲氧基恶唑,包括N-杂环取代的恶唑和空间受阻的氨基恶唑,这些是传统的形成恶唑的Ugi型反应难以获得的。此一锅法涉及内酰胺/酰胺的Ir催化部分还原,以及异氰酸酯中异氰酸酯基团的顺序化学选择性加成。
更新日期:2018-05-17
中文翻译:

铱催化的内酰胺/酰胺与异氰基乙酸盐的还原偶联反应†
对于将酰胺一锅转化为其他类型化合物的催化CC键形成方法提出了很高的要求。在此报告中,我们证明了将异氰基乙酸酯催化还原加成到叔内酰胺/酰胺上可生成5-甲氧基恶唑,包括N-杂环取代的恶唑和空间受阻的氨基恶唑,这些是传统的形成恶唑的Ugi型反应难以获得的。此一锅法涉及内酰胺/酰胺的Ir催化部分还原,以及异氰酸酯中异氰酸酯基团的顺序化学选择性加成。











































 京公网安备 11010802027423号
京公网安备 11010802027423号