当前位置:
X-MOL 学术
›
Inorg. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Structural Characterization and Photochemical Properties of Mono- and Bimetallic Cu-Mabiq Complexes
Inorganic Chemistry ( IF 4.3 ) Pub Date : 2018-05-16 00:00:00 , DOI: 10.1021/acs.inorgchem.8b00471 H. Sophia Stark 1 , Philipp J. Altmann 1 , Stephen Sproules 2 , Corinna R. Hess 1
Inorganic Chemistry ( IF 4.3 ) Pub Date : 2018-05-16 00:00:00 , DOI: 10.1021/acs.inorgchem.8b00471 H. Sophia Stark 1 , Philipp J. Altmann 1 , Stephen Sproules 2 , Corinna R. Hess 1
Affiliation
|
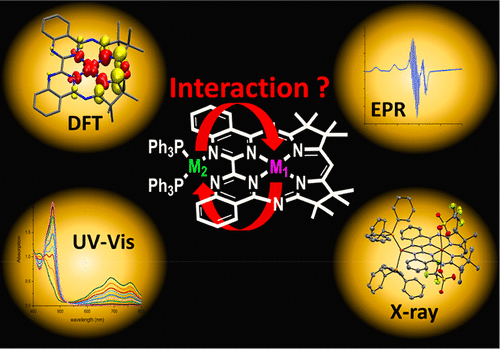
We present a series of monometallic ([Cu(Mabiq)OTf] (1) and [Cu(Mabiq)] (2)) and bimetallic copper-Mabiq complexes ([Cu2(Mabiq)(PPh3)2(OTf)2] (3) and [Cu2(Mabiq)(PPh3)2]PF6 (4)). The latter compounds contain an additional CuI center that binds in a tetrahedral fashion to the external bipyrimidine nitrogens of the macrocyclic ligand. Compounds 3 and 4 represent the first examples of bimetallic transition metal Mabiq complexes, stable both in solution and in the solid state. The structural and electronic properties of compounds 1–4 were analyzed by means of X-ray crystallography, cyclic voltammetry, and spectroscopic methods. One-electron reduced 2 and 4 consist of a CuII ion coordinated by a Mabiq ligand radical, [CuII(Mabiq•)]. Thus, both bimetallic compounds are mixed-valent with respect to the copper oxidation states. Complexes 2 and 4 can be generated photochemically, upon irradiation of 1 or 3 with visible light in the presence of a sacrificial electron donor.
中文翻译:

单金属和双金属Cu-Mabiq配合物的结构表征和光化学性质
我们提出了一系列单金属([Cu(Mabiq)OTf](1)和[Cu(Mabiq)](2))和双金属铜-Mabiq络合物([Cu 2(Mabiq)(PPh 3)2(OTf)2 ](3)和[Cu 2(Mabiq)(PPh 3)2 ] PF 6(4))。后者的化合物含有一个额外的Cu I中心,该中心以四面体的方式与大环配体的外部联嘧啶氮原子结合。化合物3和4代表在溶液和固态均稳定的双金属过渡金属Mabiq配合物的第一个实例。化合物的结构和电子性质1 - 4通过X射线晶体学,循环伏安法,和光谱法进行分析。一电子还原的2和4由与Mabiq配体自由基[Cu II(Mabiq •)]配位的Cu II离子组成。因此,两种双金属化合物相对于铜的氧化态是混合价的。配合物2和4可以在1或3的照射下以光化学方式生成 在牺牲电子供体的存在下用可见光照射。
更新日期:2018-05-16
中文翻译:

单金属和双金属Cu-Mabiq配合物的结构表征和光化学性质
我们提出了一系列单金属([Cu(Mabiq)OTf](1)和[Cu(Mabiq)](2))和双金属铜-Mabiq络合物([Cu 2(Mabiq)(PPh 3)2(OTf)2 ](3)和[Cu 2(Mabiq)(PPh 3)2 ] PF 6(4))。后者的化合物含有一个额外的Cu I中心,该中心以四面体的方式与大环配体的外部联嘧啶氮原子结合。化合物3和4代表在溶液和固态均稳定的双金属过渡金属Mabiq配合物的第一个实例。化合物的结构和电子性质1 - 4通过X射线晶体学,循环伏安法,和光谱法进行分析。一电子还原的2和4由与Mabiq配体自由基[Cu II(Mabiq •)]配位的Cu II离子组成。因此,两种双金属化合物相对于铜的氧化态是混合价的。配合物2和4可以在1或3的照射下以光化学方式生成 在牺牲电子供体的存在下用可见光照射。











































 京公网安备 11010802027423号
京公网安备 11010802027423号