当前位置:
X-MOL 学术
›
Chem. Commun.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Selective formation of heterocyclic trans-cycloalkenes by alkyne addition to a biphenylene-based phosphane/borane frustrated Lewis pair†
Chemical Communications ( IF 4.3 ) Pub Date : 2018-05-15 00:00:00 , DOI: 10.1039/c8cc03369b Jun Li 1, 2, 3, 4 , Constantin G. Daniliuc 1, 2, 3, 4 , Gerald Kehr 1, 2, 3, 4 , Gerhard Erker 1, 2, 3, 4
Chemical Communications ( IF 4.3 ) Pub Date : 2018-05-15 00:00:00 , DOI: 10.1039/c8cc03369b Jun Li 1, 2, 3, 4 , Constantin G. Daniliuc 1, 2, 3, 4 , Gerald Kehr 1, 2, 3, 4 , Gerhard Erker 1, 2, 3, 4
Affiliation
|
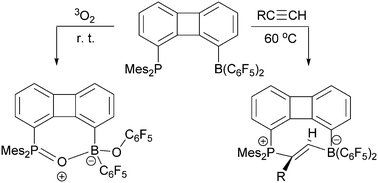
The intramolecular 1-PMes2/8-B(C6F5)2 substituted biphenylene frustrated Lewis pair 4 shows some behavior reminiscent of intermolecular FLP systems. It undergoes trans-1,2-addition to a series of 1-alkynes to give the respective heterocyclic eight-membered E-alkenes 8. The P/B FLP 4 also reacts with triplet dioxygen to yield the [P]–O–[B](OC6F5) containing oxygenation product.
中文翻译:

通过将炔烃加到联苯基膦/硼烷上受阻的路易斯对上, 选择性地形成杂环反式环烯烃†
分子内1-PMes 2 / 8-B(C 6 F 5)2取代的联苯阻抑的路易斯对4显示出一些行为,让人联想到分子间FLP系统。它经历反式-1,2-加成反应生成一系列1-炔烃,得到各自的杂环八元E-烯烃8。P / B FLP 4也与三重态双氧反应生成[P] –O– [ B](OC 6 F 5)含氧合产物。
更新日期:2018-05-15
中文翻译:

通过将炔烃加到联苯基膦/硼烷上受阻的路易斯对上, 选择性地形成杂环反式环烯烃†
分子内1-PMes 2 / 8-B(C 6 F 5)2取代的联苯阻抑的路易斯对4显示出一些行为,让人联想到分子间FLP系统。它经历反式-1,2-加成反应生成一系列1-炔烃,得到各自的杂环八元E-烯烃8。P / B FLP 4也与三重态双氧反应生成[P] –O– [ B](OC 6 F 5)含氧合产物。











































 京公网安备 11010802027423号
京公网安备 11010802027423号