当前位置:
X-MOL 学术
›
Chem. Sci.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Facile synthesis of α-aminoboronic acids from amines and potassium acyltrifluoroborates (KATs) via trifluoroborate-iminiums (TIMs)†
Chemical Science ( IF 7.6 ) Pub Date : 2018-05-16 00:00:00 , DOI: 10.1039/c8sc01486h Tomoya Shiro 1 , Anne Schuhmacher 1 , Moritz K Jackl 1 , Jeffrey W Bode 1
Chemical Science ( IF 7.6 ) Pub Date : 2018-05-16 00:00:00 , DOI: 10.1039/c8sc01486h Tomoya Shiro 1 , Anne Schuhmacher 1 , Moritz K Jackl 1 , Jeffrey W Bode 1
Affiliation
|
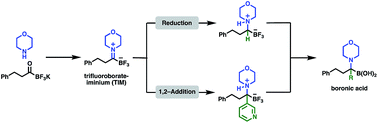
We report the facile formation of trifluoroborate-iminiums (TIMs) from potassium acyltrifluoroborates (KATs) and the transformation of TIMs to α-aminotrifluoroborates by reduction or Grignard additions. Conditions for the hydrolysis of α-aminotrifluoroborates to α-aminoboronic acids, which are important biologically active compounds, were established. This new methodology allows access to sterically demanding α-aminoboronic acids that are not easily prepared with currently available methods. This work also introduces TIMs, that can be easily prepared and handled, as a new category of functional groups that serve as precursors to valuable organic compounds.
中文翻译:

通过三氟硼酸亚胺 (TIM) 从胺和酰基三氟硼酸钾 (KAT) 轻松合成 α-氨基硼酸†
我们报道了从酰基三氟硼酸钾(KAT)容易形成三氟硼酸亚胺(TIM),以及通过还原或格氏加成将TIM转化为α-氨基三氟硼酸盐。建立了α-氨基三氟硼酸盐水解为α-氨基硼酸的条件,α-氨基硼酸是重要的生物活性化合物。这种新方法允许获得空间要求高的 α-氨基硼酸,而目前可用的方法不易制备这些α-氨基硼酸。这项工作还介绍了易于制备和处理的TIM,作为一类新的官能团,可作为有价值的有机化合物的前体。
更新日期:2018-05-16
中文翻译:

通过三氟硼酸亚胺 (TIM) 从胺和酰基三氟硼酸钾 (KAT) 轻松合成 α-氨基硼酸†
我们报道了从酰基三氟硼酸钾(KAT)容易形成三氟硼酸亚胺(TIM),以及通过还原或格氏加成将TIM转化为α-氨基三氟硼酸盐。建立了α-氨基三氟硼酸盐水解为α-氨基硼酸的条件,α-氨基硼酸是重要的生物活性化合物。这种新方法允许获得空间要求高的 α-氨基硼酸,而目前可用的方法不易制备这些α-氨基硼酸。这项工作还介绍了易于制备和处理的TIM,作为一类新的官能团,可作为有价值的有机化合物的前体。









































 京公网安备 11010802027423号
京公网安备 11010802027423号