当前位置:
X-MOL 学术
›
Chem. Sci.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Conformational landscape of the epidermal growth factor receptor kinase reveals a mutant specific allosteric pocket†
Chemical Science ( IF 7.6 ) Pub Date : 2018-05-16 00:00:00 , DOI: 10.1039/c8sc01262h Srinivasaraghavan Kannan 1, 2, 3 , Gireedhar Venkatachalam 2, 4, 5 , Hong Hwa Lim 2, 4, 5, 6, 7 , Uttam Surana 2, 4, 5, 6, 7 , Chandra Verma 1, 2, 3, 8, 9
Chemical Science ( IF 7.6 ) Pub Date : 2018-05-16 00:00:00 , DOI: 10.1039/c8sc01262h Srinivasaraghavan Kannan 1, 2, 3 , Gireedhar Venkatachalam 2, 4, 5 , Hong Hwa Lim 2, 4, 5, 6, 7 , Uttam Surana 2, 4, 5, 6, 7 , Chandra Verma 1, 2, 3, 8, 9
Affiliation
|
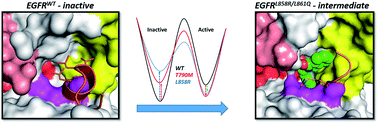
Activating mutations within the epidermal growth factor receptor (EGFR) kinase domain give rise to several cancers including Non-Small Cell Lung Cancer (NSCLC). Small molecule inhibitors targeted at these mutants have proven to be clinically successful drugs. These molecules are ATP competitive and rapidly result in the emergence of resistance. Recently Jia et al. [Nature, 2016, 534, 129–132] reported a small molecule inhibitor (called EAI045) that binds at an allosteric pocket, does not compete with ATP and displays high potency and selectivity towards certain activating mutants (L858R, T790M, L858R/T790M) of EGFR, with IC50 values ranging from 3 nM to 49 nM. We present here a study combining extensive molecular dynamics simulations with binding assays to provide a structural basis underlying the mechanism of binding of this molecule. It appears that in mutants, conformational destabilization of the short helix (that carries Leu858 in the wildtype), is key to the exposure of the allosteric pocket which otherwise is occluded by a set of sidechains including L858. We extend this hypothesis to show that a similar mechanism would enable the molecule to inhibit EGFRL861Q which is another oncogenic mutant and validate this with binding experiments. The screening of the human structural kinome revealed at least 12 other oncogenic kinases which carry at least one activating mutant in this disorder-prone region and hence would be amenable to allosteric inhibition by molecules such as EAI045. Our study characterizes a druggable allosteric pocket which appears to be specific to certain oncogenic mutants of the EGFR and holds therapeutic potential.
中文翻译:

表皮生长因子受体激酶的构象构象揭示了一个突变体特异性变构口袋†
表皮生长因子受体(EGFR)激酶结构域内的激活突变引起多种癌症,包括非小细胞肺癌(NSCLC)。针对这些突变体的小分子抑制剂已被证明是临床上成功的药物。这些分子具有ATP竞争性,并迅速导致耐药性的出现。最近贾等人。[自然,2016,534,129-132]报道的小分子抑制剂(称为EAI045),在变构袋结合,不与ATP和显示高的效力和对某些活化突变体(选择性竞争L858R,T790M,L858R / T790M)的EGFR,IC 50值范围从3 nM到49 nM。我们在这里提出了一项结合广泛的分子动力学模拟与结合测定法的研究,以提供该分子结合机理的结构基础。似乎在突变体中,短螺旋的构象失稳(在野生型中携带Leu858)是变构口袋暴露的关键,否则该变构口袋被包括L858在内的一组侧链所封闭。我们扩展了这一假设,以表明类似的机制将使该分子能够抑制EGFR L861Q这是另一个致癌突变体,并通过结合实验对此进行了验证。人类结构动因组的筛选揭示了至少12种其他致癌激酶,其在该易发疾病区域中携带至少一种活化突变体,因此将适合于诸如EAI045的分子的变构抑制。我们的研究表征了可药物化的变构口袋,该口袋似乎对某些EGFR致癌突变体具有特异性,并具有治疗潜力。
更新日期:2018-05-16
中文翻译:

表皮生长因子受体激酶的构象构象揭示了一个突变体特异性变构口袋†
表皮生长因子受体(EGFR)激酶结构域内的激活突变引起多种癌症,包括非小细胞肺癌(NSCLC)。针对这些突变体的小分子抑制剂已被证明是临床上成功的药物。这些分子具有ATP竞争性,并迅速导致耐药性的出现。最近贾等人。[自然,2016,534,129-132]报道的小分子抑制剂(称为EAI045),在变构袋结合,不与ATP和显示高的效力和对某些活化突变体(选择性竞争L858R,T790M,L858R / T790M)的EGFR,IC 50值范围从3 nM到49 nM。我们在这里提出了一项结合广泛的分子动力学模拟与结合测定法的研究,以提供该分子结合机理的结构基础。似乎在突变体中,短螺旋的构象失稳(在野生型中携带Leu858)是变构口袋暴露的关键,否则该变构口袋被包括L858在内的一组侧链所封闭。我们扩展了这一假设,以表明类似的机制将使该分子能够抑制EGFR L861Q这是另一个致癌突变体,并通过结合实验对此进行了验证。人类结构动因组的筛选揭示了至少12种其他致癌激酶,其在该易发疾病区域中携带至少一种活化突变体,因此将适合于诸如EAI045的分子的变构抑制。我们的研究表征了可药物化的变构口袋,该口袋似乎对某些EGFR致癌突变体具有特异性,并具有治疗潜力。











































 京公网安备 11010802027423号
京公网安备 11010802027423号