Journal of Fluorine Chemistry ( IF 1.7 ) Pub Date : 2018-05-15 , DOI: 10.1016/j.jfluchem.2018.05.004 Viacheslav Petrov
|
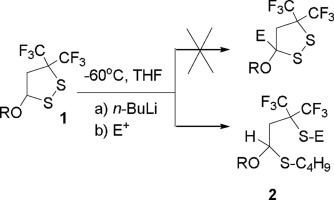
The reaction of 3,3-bis(trifluoromethyl)-5-alkoxy-1,2-dithiolanes (RO= OEt or OBu-n) proceeds through thiophilic attack of BuLi leading to the formation of ring-opened product BuSCH(OR)CH2C(CF3)2SLi, which can be converted into the corresponding thiols or sulfides by the reaction with H+ or alkyl halide. Interaction of 3,3-bis(trifluoromethyl)-5-n-butoxy-1,2-dithiolane with CF3Si(CH3)3 resulted in unusual reaction leading to the formation of CF3SCH(OBu)CH2C(CF3)=CF2 (7). The treatment of 7 with CsF in the presence pentafluoropyridine led to equimolar mixture of 2-n-butoxy-1,1-bis(trifluoromethyl)cyclopropane and 4-(trifluoromethylthio)tetrafluoropyridine (as a result of interception of liberated in this process CF3S- anion by pentafluoropyridine).
中文翻译:

3,3-双(三氟甲基)-5-烷氧基-1,2-二硫杂环戊烷在亲核试剂作用下的亲硫性开环反应
3,3-双(三氟甲基)-5-烷氧基-1,2-二硫杂环戊烷(RO = OEt或OBu-n)的反应通过BuLi的亲硫性攻击进行,导致开环产物BuSCH(OR)CH的形成2 C(CF 3)2 SLi,可通过与H +或烷基卤化物反应转化为相应的硫醇或硫化物。3,3-双(三氟甲基)-5-正丁氧基-1,2-二硫杂环戊烷与CF 3 Si(CH 3)3的相互作用导致异常反应,导致CF 3 SCH(OBu)CH 2 C( CF 3)= CF 2(7)。治疗7在存在五氟吡啶的条件下用CsF生成2-正丁氧基-1,1-双(三氟甲基)环丙烷和4-(三氟甲硫基)四氟吡啶的等摩尔混合物(由于在此过程中截获了游离的CF 3 S-阴离子五氟吡啶)。









































 京公网安备 11010802027423号
京公网安备 11010802027423号