当前位置:
X-MOL 学术
›
Chem. Bio. Drug Des.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Synthesis and biological evaluation of novel N‐aryl‐ω‐(benzoazol‐2‐yl)‐sulfanylalkanamides as dual inhibitors of α‐glucosidase and protein tyrosine phosphatase 1B
Chemical Biology & Drug Design ( IF 3.2 ) Pub Date : 2018-06-13 , DOI: 10.1111/cbdd.13331 Mei-Yan Wang 1 , Xian-Chao Cheng 2 , Xiu-Bo Chen 2, 3 , Yu Li 2 , Lan-Lan Zang 2 , Yu-Qing Duan 2 , Ming-Zhu Chen 4 , Peng Yu 4 , Hua Sun 4 , Run-Ling Wang 2
Chemical Biology & Drug Design ( IF 3.2 ) Pub Date : 2018-06-13 , DOI: 10.1111/cbdd.13331 Mei-Yan Wang 1 , Xian-Chao Cheng 2 , Xiu-Bo Chen 2, 3 , Yu Li 2 , Lan-Lan Zang 2 , Yu-Qing Duan 2 , Ming-Zhu Chen 4 , Peng Yu 4 , Hua Sun 4 , Run-Ling Wang 2
Affiliation
|
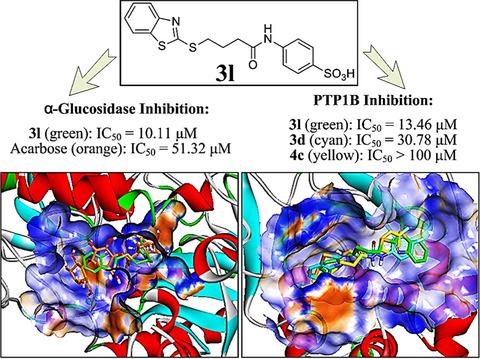
α‐Glucosidase is known to catalyze the digestion of carbohydrates and release free glucose into the digestive tract. Protein tyrosine phosphatase 1B (PTP1B) is engaged in the dephosphorylation of the insulin receptor and regulation of insulin sensitivity. Therefore, dual antagonists by targeting both α‐glucosidase and PTP1B may be potential candidates for type 2 diabetes therapy. In this work, three series of novel N‐aryl‐ω‐(benzoazol‐2‐yl)‐sulfanylalkanamides were synthesized and assayed for their α‐glucosidase and PTP1B inhibitory activities, respectively. Compound 3l, exhibiting the most effective α‐glucosidase inhibitory activity (IC50 = 10.96 μm (3l), IC50 = 51.32 μm (Acarbose), IC50 = 18.22 μm (Ursolic acid)) and potent PTP1B inhibitory activity (IC50 = 13.46 μm (3l), IC50 = 14.50 μm (Ursolic acid)), was identified as a novel dual inhibitor of α‐glucosidase and PTP1B. Furthermore, 3l is a highly selective PTP1B inhibitor because no inhibition was showed by 3l at 100 μm against PTP‐MEG2, TCPTP, SHP2, or SHP1. Subsequent kinetic analysis revealed 3l inhibited α‐glucosidase in a reversible and mixed manner. Molecular docking study indicated that hydrogen bonds, van der Waals, charge interactions and Pi‐cation interactions all contributed to affinity between 3l and α‐glucosidase/PTP1B.
中文翻译:

新型α-葡萄糖苷酶和蛋白质酪氨酸磷酸酶1B双重抑制剂N-芳基-ω-(苯并唑-2-基)-硫烷基烷基铝酰胺的合成和生物学评估
已知α-葡萄糖苷酶可催化碳水化合物的消化并将游离葡萄糖释放到消化道中。蛋白酪氨酸磷酸酶1B(PTP1B)参与胰岛素受体的去磷酸化和胰岛素敏感性的调节。因此,靶向α-葡萄糖苷酶和PTP1B的双重拮抗剂可能是2型糖尿病治疗的潜在候选者。在这项工作中,三个系列新颖的ñ -芳基- ω - (benzoazol -2-基)-sulfanylalkanamides合成并测定其分别α葡糖苷酶和PTP1B抑制活性。化合物3升,表现出最有效的α葡萄糖苷酶抑制活性(IC 50 = 10.96μ米(3升),IC 50 = 51.32μ米(阿卡波糖),IC 50 = 18.22μ米(乌索酸))和有效PTP1B的抑制活性(IC 50 = 13.46μ米(3升),IC 50 = 14.50μ米(乌索酸)),被认定为一种新型的α-葡萄糖苷酶和PTP1B双重抑制剂。此外,3升是一种高选择性PTP1B抑制剂,因为没有抑制通过显示3升在100μ米针对PTP-MEG2,TCPTP,SHP2,或SHP1。随后的动力学分析显示3l以可逆和混合的方式抑制α-葡萄糖苷酶。分子对接研究表明,氢键,范德华力,电荷相互作用和Pi-阳离子相互作用均有助于3l与α-葡萄糖苷酶/ PTP1B之间的亲和力。
更新日期:2018-06-13
中文翻译:

新型α-葡萄糖苷酶和蛋白质酪氨酸磷酸酶1B双重抑制剂N-芳基-ω-(苯并唑-2-基)-硫烷基烷基铝酰胺的合成和生物学评估
已知α-葡萄糖苷酶可催化碳水化合物的消化并将游离葡萄糖释放到消化道中。蛋白酪氨酸磷酸酶1B(PTP1B)参与胰岛素受体的去磷酸化和胰岛素敏感性的调节。因此,靶向α-葡萄糖苷酶和PTP1B的双重拮抗剂可能是2型糖尿病治疗的潜在候选者。在这项工作中,三个系列新颖的ñ -芳基- ω - (benzoazol -2-基)-sulfanylalkanamides合成并测定其分别α葡糖苷酶和PTP1B抑制活性。化合物3升,表现出最有效的α葡萄糖苷酶抑制活性(IC 50 = 10.96μ米(3升),IC 50 = 51.32μ米(阿卡波糖),IC 50 = 18.22μ米(乌索酸))和有效PTP1B的抑制活性(IC 50 = 13.46μ米(3升),IC 50 = 14.50μ米(乌索酸)),被认定为一种新型的α-葡萄糖苷酶和PTP1B双重抑制剂。此外,3升是一种高选择性PTP1B抑制剂,因为没有抑制通过显示3升在100μ米针对PTP-MEG2,TCPTP,SHP2,或SHP1。随后的动力学分析显示3l以可逆和混合的方式抑制α-葡萄糖苷酶。分子对接研究表明,氢键,范德华力,电荷相互作用和Pi-阳离子相互作用均有助于3l与α-葡萄糖苷酶/ PTP1B之间的亲和力。











































 京公网安备 11010802027423号
京公网安备 11010802027423号