当前位置:
X-MOL 学术
›
J. Med. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Design and Synthesis of Highly Potent HIV-1 Protease Inhibitors Containing Tricyclic Fused Ring Systems as Novel P2 Ligands: Structure–Activity Studies, Biological and X-ray Structural Analysis
Journal of Medicinal Chemistry ( IF 6.8 ) Pub Date : 2018-05-15 00:00:00 , DOI: 10.1021/acs.jmedchem.8b00298 Arun K Ghosh 1 , Prasanth R Nyalapatla 1 , Satish Kovela 1 , Kalapala Venkateswara Rao 1 , Margherita Brindisi 1 , Heather L Osswald 1 , Masayuki Amano 2, 3 , Manabu Aoki 2, 3, 4 , Johnson Agniswamy 5 , Yuan-Fang Wang 5 , Irene T Weber 5 , Hiroaki Mitsuya 2, 4, 6
Journal of Medicinal Chemistry ( IF 6.8 ) Pub Date : 2018-05-15 00:00:00 , DOI: 10.1021/acs.jmedchem.8b00298 Arun K Ghosh 1 , Prasanth R Nyalapatla 1 , Satish Kovela 1 , Kalapala Venkateswara Rao 1 , Margherita Brindisi 1 , Heather L Osswald 1 , Masayuki Amano 2, 3 , Manabu Aoki 2, 3, 4 , Johnson Agniswamy 5 , Yuan-Fang Wang 5 , Irene T Weber 5 , Hiroaki Mitsuya 2, 4, 6
Affiliation
|
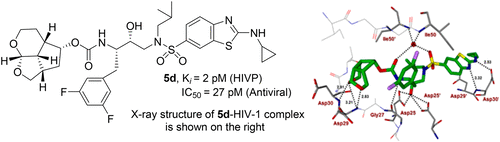
The design, synthesis, and biological evaluation of a new class of HIV-1 protease inhibitors containing stereochemically defined fused tricyclic polyethers as the P2 ligands and a variety of sulfonamide derivatives as the P2′ ligands are described. A number of ring sizes and various substituent effects were investigated to enhance the ligand–backbone interactions in the protease active site. Inhibitors 5c and 5d containing this unprecedented fused 6–5–5 ring system as the P2 ligand, an aminobenzothiazole as the P2′ ligand, and a difluorophenylmethyl as the P1 ligand exhibited exceptional enzyme inhibitory potency and maintained excellent antiviral activity against a panel of highly multidrug-resistant HIV-1 variants. The umbrella-like P2 ligand for these inhibitors has been synthesized efficiently in an optically active form using a Pauson–Khand cyclization reaction as the key step. The racemic alcohols were resolved efficiently using a lipase catalyzed enzymatic resolution. Two high resolution X-ray structures of inhibitor-bound HIV-1 protease revealed extensive interactions with the backbone atoms of HIV-1 protease and provided molecular insight into the binding properties of these new inhibitors.
中文翻译:

含有三环稠环系统作为新型 P2 配体的高效 HIV-1 蛋白酶抑制剂的设计与合成:结构-活性研究、生物学和 X 射线结构分析
描述了一类新的 HIV-1 蛋白酶抑制剂的设计、合成和生物学评价,其中包含立体化学定义的稠合三环聚醚作为 P2 配体和多种磺酰胺衍生物作为 P2' 配体。研究了多种环大小和各种取代基效应,以增强蛋白酶活性位点中的配体-主链相互作用。抑制剂5c和5d包含这种前所未有的稠合 6-5-5 环系统作为 P2 配体、氨基苯并噻唑作为 P2' 配体和二氟苯甲基作为 P1 配体,表现出卓越的酶抑制效力,并保持对一组高度多重耐药 HIV 的出色抗病毒活性-1 变体。使用 Pauson–Khand 环化反应作为关键步骤,以光学活性形式有效合成了这些抑制剂的伞状 P2 配体。使用脂肪酶催化的酶促拆分可有效拆分外消旋醇。抑制剂结合的 HIV-1 蛋白酶的两个高分辨率 X 射线结构揭示了与 HIV-1 蛋白酶骨架原子的广泛相互作用,并提供了对这些新抑制剂结合特性的分子洞察。
更新日期:2018-05-15
中文翻译:

含有三环稠环系统作为新型 P2 配体的高效 HIV-1 蛋白酶抑制剂的设计与合成:结构-活性研究、生物学和 X 射线结构分析
描述了一类新的 HIV-1 蛋白酶抑制剂的设计、合成和生物学评价,其中包含立体化学定义的稠合三环聚醚作为 P2 配体和多种磺酰胺衍生物作为 P2' 配体。研究了多种环大小和各种取代基效应,以增强蛋白酶活性位点中的配体-主链相互作用。抑制剂5c和5d包含这种前所未有的稠合 6-5-5 环系统作为 P2 配体、氨基苯并噻唑作为 P2' 配体和二氟苯甲基作为 P1 配体,表现出卓越的酶抑制效力,并保持对一组高度多重耐药 HIV 的出色抗病毒活性-1 变体。使用 Pauson–Khand 环化反应作为关键步骤,以光学活性形式有效合成了这些抑制剂的伞状 P2 配体。使用脂肪酶催化的酶促拆分可有效拆分外消旋醇。抑制剂结合的 HIV-1 蛋白酶的两个高分辨率 X 射线结构揭示了与 HIV-1 蛋白酶骨架原子的广泛相互作用,并提供了对这些新抑制剂结合特性的分子洞察。











































 京公网安备 11010802027423号
京公网安备 11010802027423号