当前位置:
X-MOL 学术
›
Inorg. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Can Electrochemical Measurements Be Used To Predict X-ray Photoelectron Spectroscopic Data? The Case of Ferrocenyl-β-Diketonato Complexes of Manganese(III)
Inorganic Chemistry ( IF 4.3 ) Pub Date : 2018-05-15 00:00:00 , DOI: 10.1021/acs.inorgchem.8b00745 Blenerhassitt E. Buitendach 1 , Elizabeth Erasmus 1 , J. W. Niemantsverdriet 2 , Jannie C. Swarts 1
Inorganic Chemistry ( IF 4.3 ) Pub Date : 2018-05-15 00:00:00 , DOI: 10.1021/acs.inorgchem.8b00745 Blenerhassitt E. Buitendach 1 , Elizabeth Erasmus 1 , J. W. Niemantsverdriet 2 , Jannie C. Swarts 1
Affiliation
|
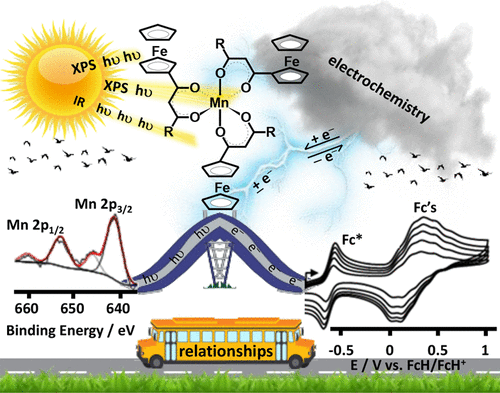
In order to better understand intramolecular communication between molecular fragments, a series of ferrocene-functionalized β-diketonato manganese(III) complexes, [Mn(FcCOCHCOR)3] with R = CF3, 1, CH3, 2, Ph = C6H5, 3, and Fc = FeII(η5-C5H4)(η5-C5H5), 4, the mixed ligand β-diketonato complex [Mn(FcCOCHCOFc)2(FcCOCHCOCH3)], 5, as well as the acac complex [Mn(CH3COCHCOCH3)3], 6, were subjected to an electrochemical and X-ray photoelectron spectroscopy (XPS) study. The ferrocenyl (FeII) and MnIII redox potentials, E°′, and photoelectron lines were sufficiently resolved in each complex to demonstrate a linear correlation between E°′ and group electronegativities of ligand R groups, χR, or ΣχR, as well as with binding energies of both the Fe 2p3/2 and Mn 2p3/2 photoelectron lines. These relationships are consistent with effective communication between molecular fragments of 1–5. From these relationships, prediction of Mn and Fe core electron binding energies in [Mn(R1COCHCOR2)3] complexes from known manganese and/or ferrocenyl redox potentials are, therefore, now possible. Ligand infrared carbonyl stretching frequencies were successfully related to binding energy as a measure of the energy required for inner-sphere reorganization. In particular it became possible to explain why, upon electrochemical oxidation or photoionization, the ferrocenyl FeII inner-shell of 1–5 needs more energy in complexes with ligands bearing electron-withdrawing (CF3) groups than in ligands bearing electron-donating groups such as ferrocenyl. The XPS determined entity Iratio (the ratio between the intensities of the satellite and main metal 2p3/2 photoelectron lines) is an indication not only of the amount of charge transferred, but also of the degree of inner-sphere reorganization. Just as for binding energy, the quantity Iratio was also found to be related to the energy requirements for the inner-sphere reorganization depicted by the vibrational frequency, vco.
中文翻译:

电化学测量可以用于预测X射线光电子能谱数据吗?锰的二茂铁基-β-二酮基配合物的情况(III)
为了更好地理解分子片段之间的分子内的通信,一系列二茂铁官能化β-二酮盐锰(III)络合物,[Mn(上FcCOCHCOR)的3 ],其中R = CF 3,1,CH 3,2中,Ph = C 6 ħ 5,3,和Fc =铁II(η 5 -C 5 H ^ 4)(η 5 -C 5 H ^ 5),4,混合配体β-二酮盐络合物[锰(FcCOCHCOFc)2(FcCOCHCOCH 3)],5以及acac配合物[Mn(CH 3 COCHCOCH 3)3 ] 6均经过电化学和X射线光电子能谱(XPS)研究。所述二茂铁基(铁II)和Mn III的氧化还原电位,ê °'和光电子线在每个复合物充分消除演示之间的线性相关ë °'和配体的R基团的基团的电负,χ - [R ,或Σχ - [R ,如以及Fe 2p 3/2和Mn 2p 3/2的结合能光电子线。这些关系是随着分子片段之间的有效通信一致1 - 5。因此,根据这些关系,现在有可能从已知的锰和/或二茂铁基氧化还原电势预测[Mn(R 1 COCHCOR 2)3 ]配合物中的Mn和Fe核电子结合能。配体红外羰基的拉伸频率已成功地与结合能相关,以作为内球重组所需能量的量度。特别地,它成为可能解释为什么,在电化学氧化或光致电离,二茂铁的Fe II的内壳层1 - 5与带有吸电子基团的配体(如二茂铁基)配位体相比,与带有吸电子基团(CF 3)的配位体需要更多的能量。XPS确定的实体I比率(卫星和主要金属2p 3/2光电子线的强度之比)不仅表示电荷转移的数量,而且还表示内部球的重组程度。就像束缚能量一样,量I比也与振动频率v co所描绘的内球重组的能量需求有关。
更新日期:2018-05-15
中文翻译:

电化学测量可以用于预测X射线光电子能谱数据吗?锰的二茂铁基-β-二酮基配合物的情况(III)
为了更好地理解分子片段之间的分子内的通信,一系列二茂铁官能化β-二酮盐锰(III)络合物,[Mn(上FcCOCHCOR)的3 ],其中R = CF 3,1,CH 3,2中,Ph = C 6 ħ 5,3,和Fc =铁II(η 5 -C 5 H ^ 4)(η 5 -C 5 H ^ 5),4,混合配体β-二酮盐络合物[锰(FcCOCHCOFc)2(FcCOCHCOCH 3)],5以及acac配合物[Mn(CH 3 COCHCOCH 3)3 ] 6均经过电化学和X射线光电子能谱(XPS)研究。所述二茂铁基(铁II)和Mn III的氧化还原电位,ê °'和光电子线在每个复合物充分消除演示之间的线性相关ë °'和配体的R基团的基团的电负,χ - [R ,或Σχ - [R ,如以及Fe 2p 3/2和Mn 2p 3/2的结合能光电子线。这些关系是随着分子片段之间的有效通信一致1 - 5。因此,根据这些关系,现在有可能从已知的锰和/或二茂铁基氧化还原电势预测[Mn(R 1 COCHCOR 2)3 ]配合物中的Mn和Fe核电子结合能。配体红外羰基的拉伸频率已成功地与结合能相关,以作为内球重组所需能量的量度。特别地,它成为可能解释为什么,在电化学氧化或光致电离,二茂铁的Fe II的内壳层1 - 5与带有吸电子基团的配体(如二茂铁基)配位体相比,与带有吸电子基团(CF 3)的配位体需要更多的能量。XPS确定的实体I比率(卫星和主要金属2p 3/2光电子线的强度之比)不仅表示电荷转移的数量,而且还表示内部球的重组程度。就像束缚能量一样,量I比也与振动频率v co所描绘的内球重组的能量需求有关。











































 京公网安备 11010802027423号
京公网安备 11010802027423号