Applied Catalysis B: Environment and Energy ( IF 20.2 ) Pub Date : 2018-05-15 , DOI: 10.1016/j.apcatb.2018.05.032 Carla di Luca , Paola Massa , Javier M. Grau , Sergio G. Marchetti , Rosa Fenoglio , Patricia Haure
|
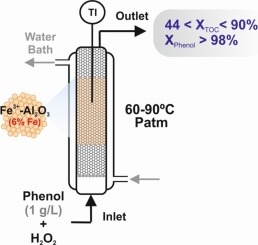
A highly dispersed Fe3+-Al2O3 catalyst (6 wt% Fe) was used for the catalytic wet hydrogen peroxide oxidation of phenol (1 g/L) in an up-flow fixed bed reactor (UFBR) under continuous operation. To enhance catalytic performance, three simple synthesis strategies were combined: two-stage impregnation of iron citrate, acid washing with CH3COOH and thermal treatment at 900 °C. Solid samples were characterized in depth by several techniques: N2 Physisorption, XRD, SEM–EDS, TEM, TGA, PZC, TPD of pyridine, XPS and Mössbauer. Peroxidation experiments were performed in an UFBR over a wide range of operating parameters in order to evaluate their influence on phenol mineralization and catalyst stability. Under selected operating condition (T = 90 °C, Wcat = 20 g, QL = 1.2 mL/min and [H2O2]:[Phenol] = 16.8), complete phenol conversion and remarkable TOC reduction of 90% were achieved, with a high H2O2 consumption efficiency (η = 76 %) and low Fe leaching (< 3 mg/L). After 70 h of usage at different steady state conditions, the catalyst retained high mineralization levels (XTOC > 70%) but the cumulative iron loss was calculated to be c.a. 20% of the initial Fe loaded in the UFBR. The catalyst was susceptible to leaching due to the accumulation of complexing intermediates such as carboxylic acids. However, acceptable iron leaching values (< 10 mg/L) were achieved when the reactor operating conditions were properly set (55% < XTOC > 80%). The presence of chelating by-products favored also the Fe redistribution inside the catalyst pellets. Nevertheless, catalyst decay in the long-term operation was mainly due to the occurrence and permanence of chelating organic acids. This process was specially promoted by the amphoteric character of the alumina-based catalyst. However, adsorbed species were promptly eliminated by calcination at 500 °C, recovering steady state conversion profiles.
中文翻译:

高度分散的Fe 3+ -Al 2 O 3,用于在连续上流固定床反应器中对苯酚进行芬顿状氧化。通过操作条件提高催化剂稳定性
在上流固定床反应器(UFBR)中,在连续操作下,将高度分散的Fe 3+ -Al 2 O 3催化剂(Fe含量为6 wt%)用于苯酚的湿式过氧化氢催化氧化(1 g / L)。为了增强催化性能,组合了三种简单的合成策略:柠檬酸铁的两步浸渍,用CH 3 COOH进行酸洗和在900°C下进行热处理。通过几种技术对固体样品进行了深度表征:N 2吡啶的物理吸收,XRD,SEM-EDS,TEM,TGA,PZC,TPD,XPS和Mössbauer。为了在UFBR中对过氧化参数进行实验,以评估其对苯酚矿化和催化剂稳定性的影响。在选定的运行条件下(T = 90°C,W cat = 20 g,Q L = 1.2 mL / min且[H 2 O 2 ]:[苯酚] = 16.8),苯酚完全转化并且TOC显着降低了90%实现了H 2 O 2的高消耗效率(η= 76%)和低Fe浸出(<3 mg / L)。在不同的稳态条件下使用70小时后,催化剂保留了较高的矿化度(X TOC大于70%),但累积的铁损量约为UFBR中初始铁含量的20%。由于络合中间体如羧酸的积累,催化剂易于浸出。但是,当适当设定反应器运行条件(55%<X TOC > 80%)时,可获得可接受的铁浸出值(<10 mg / L )。螯合副产物的存在也有利于催化剂粒料中的Fe重新分布。然而,长期运行中催化剂的降解主要归因于螯合有机酸的存在和持久性。氧化铝基催化剂的两性特性特别促进了该过程。但是,在500°C下煅烧可迅速消除吸附的物质,从而恢复稳态转化曲线。











































 京公网安备 11010802027423号
京公网安备 11010802027423号