当前位置:
X-MOL 学术
›
Org. Lett.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Synthesis of [1,2,4]Triazolo[4,3-a]piperazin-6-ones: An Approach to the Triazole-Fused Ketopiperazine Scaffold
Organic Letters ( IF 4.9 ) Pub Date : 2018-05-15 00:00:00 , DOI: 10.1021/acs.orglett.8b01112 Khoubaib Ben Haj Salah 1 , Baptiste Legrand 1 , Mathieu Bibian 1 , Emmanuel Wenger 2 , Jean-Alain Fehrentz 1 , Séverine Denoyelle 1
Organic Letters ( IF 4.9 ) Pub Date : 2018-05-15 00:00:00 , DOI: 10.1021/acs.orglett.8b01112 Khoubaib Ben Haj Salah 1 , Baptiste Legrand 1 , Mathieu Bibian 1 , Emmanuel Wenger 2 , Jean-Alain Fehrentz 1 , Séverine Denoyelle 1
Affiliation
|
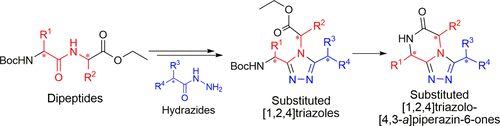
A stereoconservative synthesis to access the triazole-fused ketopiperazine (TKP) scaffold is presented. This underexplored platform offers a wide range of structural modulations with several points of diversity and chiral centers. A series of [1,2,4]triazolo[4,3-a]piperazin-6-ones was synthesized from optically pure dipeptides. The methodology was then successfully applied to access the pyrrolo[1,2-a]triazolo[3,4-c]piperazin-6-one tricycle. Importantly, the crystal structures of representative TKPs confirmed that the configuration of the chiral centers was controlled during the synthetic route and facilitated description of the orientation of the substituents depending on their nature and position on the TKP scaffold.
中文翻译:

[1,2,4] Triazolo [4,3 - a ] piperazin-6-ones的合成:一种三唑融合酮戊哌嗪支架的方法。
提出了立体保守的合成,以接近三唑融合的酮哌嗪(TKP)支架。这个开发不足的平台可提供多种结构调制,并具有多个多样性点和手性中心。从光学纯的二肽合成了一系列[1,2,4]三唑并[4,3 - a ]哌嗪-6-。该方法然后成功地应用于访问吡咯并[1,2- a ]三唑并[3,4- c ]哌嗪-6-一三轮车。重要的是,代表性TKP的晶体结构证实在合成路线中手性中心的构型是受控的,并且取决于取代基的性质和在TKP支架上的位置有利于描述取代基的取向。
更新日期:2018-05-15
中文翻译:

[1,2,4] Triazolo [4,3 - a ] piperazin-6-ones的合成:一种三唑融合酮戊哌嗪支架的方法。
提出了立体保守的合成,以接近三唑融合的酮哌嗪(TKP)支架。这个开发不足的平台可提供多种结构调制,并具有多个多样性点和手性中心。从光学纯的二肽合成了一系列[1,2,4]三唑并[4,3 - a ]哌嗪-6-。该方法然后成功地应用于访问吡咯并[1,2- a ]三唑并[3,4- c ]哌嗪-6-一三轮车。重要的是,代表性TKP的晶体结构证实在合成路线中手性中心的构型是受控的,并且取决于取代基的性质和在TKP支架上的位置有利于描述取代基的取向。









































 京公网安备 11010802027423号
京公网安备 11010802027423号