当前位置:
X-MOL 学术
›
Asian J. Org. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Functionalized Cyclopentadienyl Ligands and Their Substituent Effects on a Rhodium(III)‐Catalyzed Oxidative [4+2] Annulation of Indole‐ and Pyrrole‐1‐Carboxamides with Alkynes
Asian Journal of Organic Chemistry ( IF 2.8 ) Pub Date : 2018-06-08 , DOI: 10.1002/ajoc.201800262 Takayuki Yamada 1 , Yu Shibata 1 , Ken Tanaka 1
Asian Journal of Organic Chemistry ( IF 2.8 ) Pub Date : 2018-06-08 , DOI: 10.1002/ajoc.201800262 Takayuki Yamada 1 , Yu Shibata 1 , Ken Tanaka 1
Affiliation
|
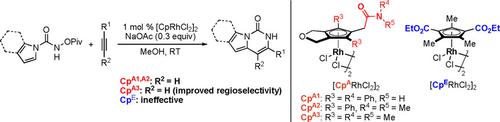
The effect of substituents on carbamoylmethyl‐cyclopentadienyl (CpA) ligands on the neutral rhodium(III)‐catalyzed oxidative [4+2] annulation of indole‐ and pyrrole‐1‐carboxamides with alkynes, in which the C−H bond cleavage is the partially rate‐limiting step, was investigated. As a result, in the reactions with terminal alkynes, a rhodium(III) complex with a dimethyl‐substituted CpA ligand (CpA3) showed high catalytic activity and improved the regioselectivity as compared to a commercially available Cp*RhIII complex. On the other hand, in reactions with internal alkynes, a rhodium(III) complex with a diphenyl‐substituted CpA ligand (CpA1 or CpA2) showed high catalytic activity, which is comparable to the activity of the Cp*RhIII complex. Interestingly, a rhodium(III) complex with an electron‐deficient di(ethoxycarbonyl)‐substituted Cp ligand (CpE) that shows high catalytic activity toward the annulation of benzamides with internal alkynes, in which the C−H cleavage is rate‐limiting, showed low catalytic activity toward the reactions with both terminal and internal alkynes. The ligand effects above provide important guidelines for the application of our CpA and CpE ligands to the rhodium(III)‐catalyzed C−H bond functionalization reactions.
中文翻译:

功能化的环戊二烯基配体及其在铑(III)催化的吲哚和吡咯-1-羧酰胺与炔烃的氧化[4 + 2]环化反应中的取代作用
取代基对中性铑(III)催化的炔烃和吡咯-1-羧酰胺与炔烃的中性铑(III)催化氧化[4 + 2]环合反应上的氨基甲酰基甲基-环戊二烯基(Cp A)配体的影响研究了部分限速步骤。结果,在与末端炔烃的反应中,与市售Cp * Rh III配合物相比,具有二甲基取代的Cp A配体(Cp A3)的铑(III)配合物显示出高催化活性并改善了区域选择性。另一方面,在与内部炔烃反应时,铑(III)与二苯基取代的Cp A配体(Cp A1或Cp A2)显示出高催化活性,与Cp * Rh III配合物的活性相当。有趣的是,具有缺电子的二(乙氧基羰基)取代的Cp配体(Cp E)的铑(III)配合物对苯甲酰胺与内部炔烃的环合显示出高催化活性,其中CH裂解是限速的,对末端和内部炔烃的反应均显示出较低的催化活性。上面的配体效应为将我们的Cp A和Cp E配体应用到铑(III)催化的C-H键官能化反应中提供了重要的指导。
更新日期:2018-06-08
中文翻译:

功能化的环戊二烯基配体及其在铑(III)催化的吲哚和吡咯-1-羧酰胺与炔烃的氧化[4 + 2]环化反应中的取代作用
取代基对中性铑(III)催化的炔烃和吡咯-1-羧酰胺与炔烃的中性铑(III)催化氧化[4 + 2]环合反应上的氨基甲酰基甲基-环戊二烯基(Cp A)配体的影响研究了部分限速步骤。结果,在与末端炔烃的反应中,与市售Cp * Rh III配合物相比,具有二甲基取代的Cp A配体(Cp A3)的铑(III)配合物显示出高催化活性并改善了区域选择性。另一方面,在与内部炔烃反应时,铑(III)与二苯基取代的Cp A配体(Cp A1或Cp A2)显示出高催化活性,与Cp * Rh III配合物的活性相当。有趣的是,具有缺电子的二(乙氧基羰基)取代的Cp配体(Cp E)的铑(III)配合物对苯甲酰胺与内部炔烃的环合显示出高催化活性,其中CH裂解是限速的,对末端和内部炔烃的反应均显示出较低的催化活性。上面的配体效应为将我们的Cp A和Cp E配体应用到铑(III)催化的C-H键官能化反应中提供了重要的指导。











































 京公网安备 11010802027423号
京公网安备 11010802027423号