当前位置:
X-MOL 学术
›
ChemSusChem
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
A Two‐Step Process for the Synthesis of Hydroxytyrosol
ChemSusChem ( IF 8.4 ) Pub Date : 2018-06-11 , DOI: 10.1002/cssc.201800684 Paolo Ziosi 1, 2 , Claudio Paolucci 1 , Francesco Santarelli 1 , Tommaso Tabanelli 1 , Sauro Passeri 3 , Fabrizio Cavani 1, 2 , Paolo Righi 1
ChemSusChem ( IF 8.4 ) Pub Date : 2018-06-11 , DOI: 10.1002/cssc.201800684 Paolo Ziosi 1, 2 , Claudio Paolucci 1 , Francesco Santarelli 1 , Tommaso Tabanelli 1 , Sauro Passeri 3 , Fabrizio Cavani 1, 2 , Paolo Righi 1
Affiliation
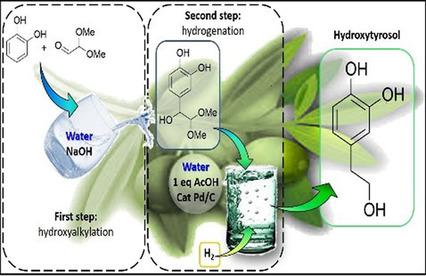
|
A new process for the synthesis of hydroxytyrosol (3,4‐dihydroxyphenylethanol), the most powerful natural antioxidant currently known, by means of a two‐step approach is reported. Catechol is first reacted with 2,2‐dimethoxyacetaldehyde in basic aqueous medium to produce the corresponding mandelic derivative with >90 % conversion of the limiting reactant and about 70 % selectivity to the desired para‐hydroxyalkylated compound. Thereafter, the intermediate is hydrogenated to hydroxytyrosol by using a Pd/C catalyst, with total conversion of the mandelic derivative and 68 % selectivity. This two‐step process is the first example of a synthetic pathway for hydroxytyrosol that does not involve the use of halogenated components or reduction methodologies that produce stoichiometric waste. It also avoids the complex procedure currently used for hydroxytyrosol purification when it is extracted from wastewater of olive oil production.
中文翻译:

合成羟基酪醇的两步法
报道了一种通过两步法合成羟基酪醇(3,4-二羟基苯基乙醇)(一种目前已知的最强大的天然抗氧化剂)的新方法。首先将邻苯二酚与2,2-二甲氧基乙醛在碱性水性介质中反应,以产生相应的扁桃衍生物,其中极限反应物的转化率> 90%,对所需对位化合物的选择性约为70%羟烷基化的化合物。此后,通过使用Pd / C催化剂将中间体氢化成羟基酪醇,其中扁桃体衍生物的总转化率和68%的选择性。这个分两步的过程是羟基酪醇合成途径的第一个例子,它不涉及使用卤化组分或产生化学计量废物的还原方法。当从橄榄油生产废水中提取羟基酪醇时,它还避免了目前用于羟基酪醇纯化的复杂程序。
更新日期:2018-06-11
中文翻译:

合成羟基酪醇的两步法
报道了一种通过两步法合成羟基酪醇(3,4-二羟基苯基乙醇)(一种目前已知的最强大的天然抗氧化剂)的新方法。首先将邻苯二酚与2,2-二甲氧基乙醛在碱性水性介质中反应,以产生相应的扁桃衍生物,其中极限反应物的转化率> 90%,对所需对位化合物的选择性约为70%羟烷基化的化合物。此后,通过使用Pd / C催化剂将中间体氢化成羟基酪醇,其中扁桃体衍生物的总转化率和68%的选择性。这个分两步的过程是羟基酪醇合成途径的第一个例子,它不涉及使用卤化组分或产生化学计量废物的还原方法。当从橄榄油生产废水中提取羟基酪醇时,它还避免了目前用于羟基酪醇纯化的复杂程序。



























 京公网安备 11010802027423号
京公网安备 11010802027423号