当前位置:
X-MOL 学术
›
ChemSusChem
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Towards Hydrogen Storage through an Efficient Ruthenium‐Catalyzed Dehydrogenation of Formic Acid
ChemSusChem ( IF 7.5 ) Pub Date : 2018-06-14 , DOI: 10.1002/cssc.201800408 Zhuo Xin 1 , Jiahui Zhang 1 , Katerina Sordakis 2 , Matthias Beller 3 , Chen-Xia Du 4 , Gabor Laurenczy 2 , Yuehui Li 1
ChemSusChem ( IF 7.5 ) Pub Date : 2018-06-14 , DOI: 10.1002/cssc.201800408 Zhuo Xin 1 , Jiahui Zhang 1 , Katerina Sordakis 2 , Matthias Beller 3 , Chen-Xia Du 4 , Gabor Laurenczy 2 , Yuehui Li 1
Affiliation
|
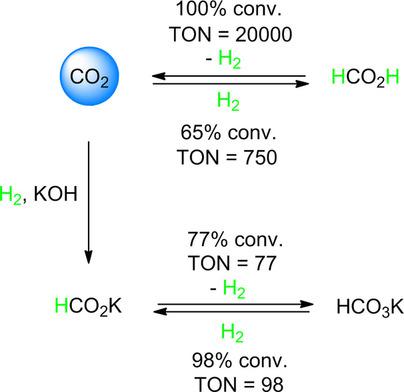
Hydrogen is of fundamental importance for the construction of modern clean‐energy supply systems. In this context, the catalytic dehydrogenation of formic acid (FA) is a convenient method to generate H2 gas from an easily available liquid. One of the issues associated with current catalytic dehydrogenation systems is insufficient stability. Here, we present a robust and recyclable system for FA dehydrogenation by combining a ruthenium 1,1,1‐tris(diphenylphosphinomethyl)ethane complex and aluminum trifluoromethanesulfonate (Al(OTf)3). This robust system allows steady H2 production under pressure and recycling for an additional 14 runs without any apparent loss of activity (turnover frequencies up to 1920 h−1, turnover numbers up to 20 000). Notably, the catalyst can also be used for the dehydrogenation of formates and the reverse hydrogenation of bicarbonates and CO2.
中文翻译:

通过有效的钌催化甲酸脱氢制氢
氢对于建设现代清洁能源供应系统至关重要。在这种情况下,甲酸(FA)的催化脱氢是一种从易得的液体中生成H 2气体的便捷方法。与当前的催化脱氢系统相关的问题之一是稳定性不足。在这里,我们通过结合钌1,1,1-三(二苯基膦甲基)乙烷络合物和三氟甲磺酸铝(Al(OTf)3)提出了一种健壮且可回收的FA脱氢系统。这个强大的系统允许在压力下稳定产生H 2,并可以再循环14次而没有任何明显的活动损失(周转频率高达1920 h -1,营业额高达2万)。值得注意的是,该催化剂还可以用于甲酸酯的脱氢以及碳酸氢盐和CO 2的逆氢化。
更新日期:2018-06-14
中文翻译:

通过有效的钌催化甲酸脱氢制氢
氢对于建设现代清洁能源供应系统至关重要。在这种情况下,甲酸(FA)的催化脱氢是一种从易得的液体中生成H 2气体的便捷方法。与当前的催化脱氢系统相关的问题之一是稳定性不足。在这里,我们通过结合钌1,1,1-三(二苯基膦甲基)乙烷络合物和三氟甲磺酸铝(Al(OTf)3)提出了一种健壮且可回收的FA脱氢系统。这个强大的系统允许在压力下稳定产生H 2,并可以再循环14次而没有任何明显的活动损失(周转频率高达1920 h -1,营业额高达2万)。值得注意的是,该催化剂还可以用于甲酸酯的脱氢以及碳酸氢盐和CO 2的逆氢化。











































 京公网安备 11010802027423号
京公网安备 11010802027423号