当前位置:
X-MOL 学术
›
Chem. Asian J.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Nickel‐Catalyzed Regioselective C(2)−H Difluoroalkylation of Indoles with Difluoroalkyl Bromides
Chemistry - An Asian Journal ( IF 3.5 ) Pub Date : 2018-06-14 , DOI: 10.1002/asia.201800504 Vineeta Soni 1 , Dipesh M. Sharma 1 , Benudhar Punji 1
Chemistry - An Asian Journal ( IF 3.5 ) Pub Date : 2018-06-14 , DOI: 10.1002/asia.201800504 Vineeta Soni 1 , Dipesh M. Sharma 1 , Benudhar Punji 1
Affiliation
|
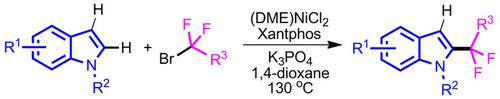
Regioselective C(2)−H difluoroalkylation of C‐3 unsubstituted indoles with commonly available fluoroalkyl bromides is successfully achieved employing a simple nickel catalyst system, (DME)NiCl2/Xantphos. This methodology shows excellent regioselectivity and exhibits a broad substrate scope. Various functional groups, such as ‐OMe, ‐F, and ‐Br, are tolerated on the indole backbone to give the difluoroalkylated products in moderate to good yields. Preliminary mechanistic findings demonstrate that the reaction is homogeneous in nature and involves a radical manifold. Synthetic utility of this nickel‐catalyzed method is demonstrated by synthesizing melatonin receptor antagonist Luzindole derivative.
中文翻译:

镍催化的吲哚与二氟烷基溴的区域选择性C(2)-H二氟烷基化
使用简单的镍催化剂体系(DME)NiCl 2 / Xantphos成功地实现了C-3未取代的吲哚与常用的氟代烷基溴的区域选择性C(2)-H二氟烷基化。该方法显示出极好的区域选择性,并显示出较宽的底物范围。在吲哚主链上可以耐受各种官能团,例如OMe,-F和-Br,从而以中等至良好的收率得到二氟烷基化产物。初步的机理发现表明,该反应本质上是均相的,并且涉及自由基。合成褪黑激素受体拮抗剂Luzindole衍生物证明了这种镍催化方法的合成效用。
更新日期:2018-06-14
中文翻译:

镍催化的吲哚与二氟烷基溴的区域选择性C(2)-H二氟烷基化
使用简单的镍催化剂体系(DME)NiCl 2 / Xantphos成功地实现了C-3未取代的吲哚与常用的氟代烷基溴的区域选择性C(2)-H二氟烷基化。该方法显示出极好的区域选择性,并显示出较宽的底物范围。在吲哚主链上可以耐受各种官能团,例如OMe,-F和-Br,从而以中等至良好的收率得到二氟烷基化产物。初步的机理发现表明,该反应本质上是均相的,并且涉及自由基。合成褪黑激素受体拮抗剂Luzindole衍生物证明了这种镍催化方法的合成效用。











































 京公网安备 11010802027423号
京公网安备 11010802027423号