European Journal of Medicinal Chemistry ( IF 6.0 ) Pub Date : 2018-05-14 , DOI: 10.1016/j.ejmech.2018.05.019 Andrea Chicca , Chiara Arena , Simone Bertini , Francesca Gado , Elena Ciaglia , Mario Abate , Maria Digiacomo , Margherita Lapillo , Giulio Poli , Maurizio Bifulco , Marco Macchia , Tiziano Tuccinardi , Jürg Gertsch , Clementina Manera
|
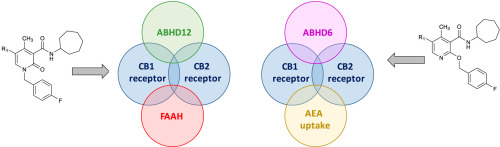
The endocannabinoid system (ECS) represents one of the major neuromodulatory systems involved in different physiological and pathological processes. Multi-target compounds exert their activities by acting via multiple mechanisms of action and represent a promising pharmacological modulation of the ECS. In this work we report 4-substituted and 4,5-disubstituted 1,2-dihydro-2-oxo-pyridine-3-carboxamide derivatives with a broad spectrum of affinity and functional activity towards both cannabinoid receptors and additional effects on the main components of the ECS. In particular compound B3 showed high affinity for CB1R (Ki = 23.1 nM, partial agonist) and CB2R (Ki = 6.9 nM, inverse agonist) and also significant inhibitory activity (IC50 = 70 nM) on FAAH with moderate inhibition of ABHD12 (IC50 = 2.5 μΜ). Compounds B4, B5 and B6 that act as full agonists at CB1R and as partial agonists (B5 and B6) or antagonist (B4) at CB2R, exhibited an additional multi-target property by inhibiting anandamide uptake with sub-micromolar IC50 values (0.28–0.62 μΜ). The best derivatives showed cytotoxic activity on U937 lymphoblastoid cells. Finally, molecular docking analysis carried out on the three-dimensional structures of CB1R and CB2R and of FAAH allowed to rationalize the structure-activity relationships of this series of compounds.
中文翻译:

内源性大麻素系统中1,2-二氢-2-氧代吡啶-3-羧酰胺的多药理学特征
内源性大麻素系统(ECS)代表参与不同生理和病理过程的主要神经调节系统之一。多靶标化合物通过多种作用机理发挥作用,代表了ECS的有希望的药理学调节作用。在这项工作中,我们报告了4-取代的和4,5-二取代的1,2-二氢-2-氧代吡啶-3-羧酰胺衍生物,对大麻素受体具有广泛的亲和力和功能活性,并且对主要成分具有附加作用ECS。特别是化合物B3对CB1R(K i = 23.1 nM,部分激动剂)和CB2R(K i = 6.9 nM,反向激动剂)表现出高亲和力,并且还具有显着的抑制活性(IC50 = 70 nM)对FAAH具有中等程度的ABHD12抑制作用(IC 50 = 2.5μM)。化合物B4,B5和B6在CB1R处起完全激动剂的作用,在CB2R处起部分激动剂(B5和B6)或拮抗剂(B4)的作用,通过抑制亚甲酰胺的摄取(亚微摩尔IC 50值为0.28 ),表现出额外的多目标特性。–0.62μM)。最好的衍生物对U937淋巴母细胞具有细胞毒活性。最后,对CB1R和CB2R以及FAAH的三维结构进行了分子对接分析,从而合理化了该系列化合物的构效关系。











































 京公网安备 11010802027423号
京公网安备 11010802027423号