当前位置:
X-MOL 学术
›
ChemBioChem
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
The Alkylquinolone Repertoire of Pseudomonas aeruginosa is Linked to Structural Flexibility of the FabH-like 2-Heptyl-3-hydroxy-4(1H)-quinolone (PQS) Biosynthesis Enzyme PqsBC.
ChemBioChem ( IF 3.2 ) Pub Date : 2018-06-22 , DOI: 10.1002/cbic.201800153 Florian Witzgall 1 , Tobias Depke 2 , Michael Hoffmann 3, 4 , Martin Empting 4, 5 , Mark Brönstrup 2 , Rolf Müller 3, 4 , Wulf Blankenfeldt 1, 6
ChemBioChem ( IF 3.2 ) Pub Date : 2018-06-22 , DOI: 10.1002/cbic.201800153 Florian Witzgall 1 , Tobias Depke 2 , Michael Hoffmann 3, 4 , Martin Empting 4, 5 , Mark Brönstrup 2 , Rolf Müller 3, 4 , Wulf Blankenfeldt 1, 6
Affiliation
|
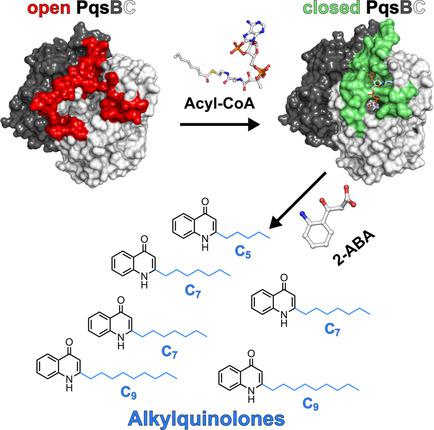
Pseudomonas aeruginosa is a bacterial pathogen that causes life-threatening infections in immunocompromised patients. It produces a large armory of saturated and mono-unsaturated 2-alkyl-4(1H)-quinolones (AQs) and AQ N-oxides (AQNOs) that serve as signaling molecules to control the production of virulence factors and that are involved in membrane vesicle formation and iron chelation; furthermore, they also have, for example, antibiotic properties. It has been shown that the β-ketoacyl-acyl-carrier protein synthase III (FabH)-like heterodimeric enzyme PqsBC catalyzes the last step in the biosynthesis of the most abundant AQ congener, 2-heptyl-4(1H)-quinolone (HHQ), by condensing octanoyl-coenzyme A (CoA) with 2-aminobenzoylacetate (2-ABA), but the basis for the large number of other AQs/AQNOs produced by P. aeruginosa is not known. Here, we demonstrate that PqsBC uses different medium-chain acyl-CoAs to produce various saturated AQs/AQNOs and that it also biosynthesizes mono-unsaturated congeners. Further, we determined the structures of PqsBC in four different crystal forms at 1.5 to 2.7 Å resolution. Together with a previous report, the data reveal that PqsBC adopts open, intermediate, and closed conformations that alter the shape of the acyl-binding cavity and explain the promiscuity of PqsBC. The different conformations also allow us to propose a model for structural transitions that accompany the catalytic cycle of PqsBC that might have broader implications for other FabH-enzymes, for which such structural transitions have been postulated but have never been observed.
中文翻译:

铜绿假单胞菌的烷基喹诺酮库与FabH样2-庚基-3-羟基-4(1H)-喹诺酮(PQS)生物合成酶PqsBC的结构柔性有关。
铜绿假单胞菌是一种细菌病原体,可在免疫功能低下的患者中造成威胁生命的感染。它产生大量的饱和和单不饱和2-烷基-4(1H)-喹诺酮(AQs)和AQ N-氧化物(AQNOs)军械库,它们充当信号分子来控制毒力因子的产生,并参与膜囊泡形成和铁螯合;此外,它们还具有例如抗生素特性。已经显示,β-酮酰基-酰基-载体蛋白合酶III(FabH)样异二聚酶PqsBC催化了最丰富的AQ同源物2-庚基-4(1H)-喹诺酮(HHQ)的生物合成的最后一步。 ),通过将辛酰基辅酶A(CoA)与2-氨基苯甲酰乙酸酯(2-ABA)进行缩合,但是由铜绿假单胞菌产生的大量其他AQ / AQNO的基础尚不清楚。这里,我们证明PqsBC使用不同的中链酰基CoA来产生各种饱和AQ / AQNO,并且它还可以生物合成单不饱和同类物。此外,我们以1.5到2.7Å的分辨率确定了四种不同晶体形式的PqsBC的结构。连同先前的报告,数据显示PqsBC采用开放,中间和封闭的构象,这些构象改变了酰基结合腔的形状并解释了PqsBC的滥交情况。不同的构象也使我们能够为PqsBC催化循环所伴随的结构转变提出一个模型,该模型可能对其他FabH酶具有更广泛的意义,但对于这种结构转变,我们已经假定但从未观察到。
更新日期:2018-06-22
中文翻译:

铜绿假单胞菌的烷基喹诺酮库与FabH样2-庚基-3-羟基-4(1H)-喹诺酮(PQS)生物合成酶PqsBC的结构柔性有关。
铜绿假单胞菌是一种细菌病原体,可在免疫功能低下的患者中造成威胁生命的感染。它产生大量的饱和和单不饱和2-烷基-4(1H)-喹诺酮(AQs)和AQ N-氧化物(AQNOs)军械库,它们充当信号分子来控制毒力因子的产生,并参与膜囊泡形成和铁螯合;此外,它们还具有例如抗生素特性。已经显示,β-酮酰基-酰基-载体蛋白合酶III(FabH)样异二聚酶PqsBC催化了最丰富的AQ同源物2-庚基-4(1H)-喹诺酮(HHQ)的生物合成的最后一步。 ),通过将辛酰基辅酶A(CoA)与2-氨基苯甲酰乙酸酯(2-ABA)进行缩合,但是由铜绿假单胞菌产生的大量其他AQ / AQNO的基础尚不清楚。这里,我们证明PqsBC使用不同的中链酰基CoA来产生各种饱和AQ / AQNO,并且它还可以生物合成单不饱和同类物。此外,我们以1.5到2.7Å的分辨率确定了四种不同晶体形式的PqsBC的结构。连同先前的报告,数据显示PqsBC采用开放,中间和封闭的构象,这些构象改变了酰基结合腔的形状并解释了PqsBC的滥交情况。不同的构象也使我们能够为PqsBC催化循环所伴随的结构转变提出一个模型,该模型可能对其他FabH酶具有更广泛的意义,但对于这种结构转变,我们已经假定但从未观察到。



























 京公网安备 11010802027423号
京公网安备 11010802027423号