当前位置:
X-MOL 学术
›
ChemBioChem
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
A Defined and Flexible Pocket Explains Aryl Substrate Promiscuity of the Cahuitamycin Starter Unit-Activating Enzyme CahJ.
ChemBioChem ( IF 2.6 ) Pub Date : 2018-06-21 , DOI: 10.1002/cbic.201800233 Ashootosh Tripathi 1, 2 , Sung Ryeol Park 1, 3 , Andrew P Sikkema 1, 4, 5 , Hyo Je Cho 6 , Jianfeng Wu 7 , Brian Lee 1 , Chuanwu Xi 7 , Janet L Smith 1, 4 , David H Sherman 1, 2, 8, 9
ChemBioChem ( IF 2.6 ) Pub Date : 2018-06-21 , DOI: 10.1002/cbic.201800233 Ashootosh Tripathi 1, 2 , Sung Ryeol Park 1, 3 , Andrew P Sikkema 1, 4, 5 , Hyo Je Cho 6 , Jianfeng Wu 7 , Brian Lee 1 , Chuanwu Xi 7 , Janet L Smith 1, 4 , David H Sherman 1, 2, 8, 9
Affiliation
|
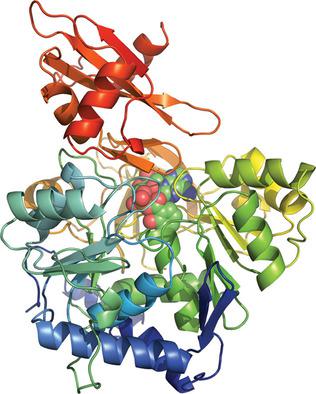
Cahuitamycins are biofilm inhibitors assembled by a convergent nonribosomal peptide synthetase pathway. Previous genetic analysis indicated that a discrete enzyme, CahJ, serves as a gatekeeper for cahuitamycin structural diversification. Here, the CahJ protein was probed structurally and functionally to guide the formation of new analogues by mutasynthetic studies. This analysis enabled the in vivo production of a new cahuitamycin congener through targeted precursor incorporation.
中文翻译:

明确且灵活的口袋解释了卡维他霉素起始单元激活酶 CahJ 的芳基底物混杂性。
卡维他霉素是通过会聚非核糖体肽合成酶途径组装的生物膜抑制剂。先前的遗传分析表明,一种离散酶 CahJ 是卡维他霉素结构多样化的看门人。在这里,我们对 CahJ 蛋白的结构和功能进行了探讨,以通过突变合成研究指导新类似物的形成。该分析使得能够通过靶向前体掺入在体内生产新的卡维他霉素同系物。
更新日期:2018-06-21
中文翻译:

明确且灵活的口袋解释了卡维他霉素起始单元激活酶 CahJ 的芳基底物混杂性。
卡维他霉素是通过会聚非核糖体肽合成酶途径组装的生物膜抑制剂。先前的遗传分析表明,一种离散酶 CahJ 是卡维他霉素结构多样化的看门人。在这里,我们对 CahJ 蛋白的结构和功能进行了探讨,以通过突变合成研究指导新类似物的形成。该分析使得能够通过靶向前体掺入在体内生产新的卡维他霉素同系物。











































 京公网安备 11010802027423号
京公网安备 11010802027423号