当前位置:
X-MOL 学术
›
Angew. Chem. Int. Ed.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Effective Assignment of α2,3/α2,6‐Sialic Acid Isomers by LC‐MS/MS‐Based Glycoproteomics
Angewandte Chemie International Edition ( IF 16.1 ) Pub Date : 2018-06-20 , DOI: 10.1002/anie.201803540 Christian Pett 1, 2 , Waqas Nasir 3, 4 , Carina Sihlbom 5 , Britt-Marie Olsson 5 , Vanessa Caixeta 1 , Manuel Schorlemer 1, 2 , René P. Zahedi 1, 6 , Göran Larson 3 , Jonas Nilsson 3 , Ulrika Westerlind 1, 2
Angewandte Chemie International Edition ( IF 16.1 ) Pub Date : 2018-06-20 , DOI: 10.1002/anie.201803540 Christian Pett 1, 2 , Waqas Nasir 3, 4 , Carina Sihlbom 5 , Britt-Marie Olsson 5 , Vanessa Caixeta 1 , Manuel Schorlemer 1, 2 , René P. Zahedi 1, 6 , Göran Larson 3 , Jonas Nilsson 3 , Ulrika Westerlind 1, 2
Affiliation
|
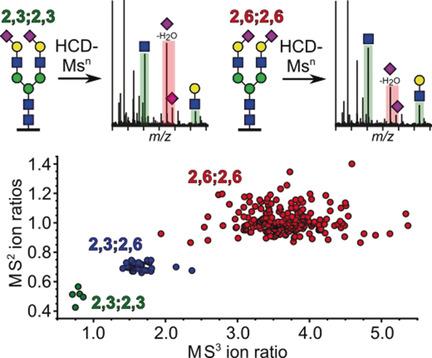
Distinct structural changes of the α2,3/α2,6‐sialic acid glycosidic linkages on glycoproteins are of importance in cancer biology, inflammatory diseases, and virus tropism. Current glycoproteomic methodologies are, however, not amenable toward high‐throughput characterization of sialic acid isomers. To enable such assignments, a mass spectrometry method utilizing synthetic model glycopeptides for the analysis of oxonium ion intensity ratios was developed. This method was successfully applied in large‐scale glycoproteomics, thus allowing the site‐specific structural characterization of sialic acid isomers.
中文翻译:

基于LC-MS / MS的糖体组学对α2,3/α2,6-唾液酸异构体的有效分配
糖蛋白上α2,3/α2,6-唾液酸糖苷键的明显结构变化在癌症生物学,炎性疾病和病毒嗜性中很重要。然而,当前的糖蛋白组学方法不适用于唾液酸异构体的高通量表征。为了进行这种分配,开发了一种利用合成模型糖肽分析氧离子强度比的质谱方法。该方法已成功应用于大规模的糖蛋白组学研究,因此可以进行唾液酸异构体的位点特异性结构表征。
更新日期:2018-06-20
中文翻译:

基于LC-MS / MS的糖体组学对α2,3/α2,6-唾液酸异构体的有效分配
糖蛋白上α2,3/α2,6-唾液酸糖苷键的明显结构变化在癌症生物学,炎性疾病和病毒嗜性中很重要。然而,当前的糖蛋白组学方法不适用于唾液酸异构体的高通量表征。为了进行这种分配,开发了一种利用合成模型糖肽分析氧离子强度比的质谱方法。该方法已成功应用于大规模的糖蛋白组学研究,因此可以进行唾液酸异构体的位点特异性结构表征。









































 京公网安备 11010802027423号
京公网安备 11010802027423号