当前位置:
X-MOL 学术
›
Org. Chem. Front.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Insights into the NHC-catalyzed cascade Michael/aldol/lactamization reaction: mechanism and origin of stereoselectivity†
Organic Chemistry Frontiers ( IF 4.6 ) Pub Date : 2018-05-14 00:00:00 , DOI: 10.1039/c8qo00398j Yang Wang 1, 2, 3, 4 , Shou-Ren Zhang 3, 5, 6, 7, 8 , Yanyan Wang 3, 4, 9, 10 , Ling-Bo Qu 3, 4, 9, 10 , Donghui Wei 3, 4, 9, 10
Organic Chemistry Frontiers ( IF 4.6 ) Pub Date : 2018-05-14 00:00:00 , DOI: 10.1039/c8qo00398j Yang Wang 1, 2, 3, 4 , Shou-Ren Zhang 3, 5, 6, 7, 8 , Yanyan Wang 3, 4, 9, 10 , Ling-Bo Qu 3, 4, 9, 10 , Donghui Wei 3, 4, 9, 10
Affiliation
|
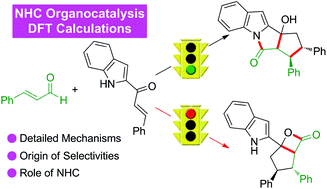
The detailed mechanism and origin of stereoselectivity of the N-heterocyclic carbene (NHC)-catalyzed cascade Michael/aldol/lactamization reaction of enals and indole-derived enones have been investigated in theory. Based on the computational results, the reaction contains seven steps, including the nucleophilic addition of NHC to enal, the intramolecular proton transfer for the formation of a Breslow intermediate, the intermolecular Michael addition reaction of indole-derived α,β-unsaturated ketone with the Breslow intermediate, the subsequent [1,6]-proton transfer to afford the enolate intermediate, the intramolecular aldol reaction coupled with the proton transfer process, the intramolecular lactamization reaction, and the release of NHC catalyst from the product. Moreover, the C–C bond formation step involved in the intermolecular Michael addition reaction was identified to be the stereoselectivity-determining step, and the RS-configured product was generated preferentially. In addition, the NCI analysis indicates that the more effective C–H⋯π and π⋯π interactions contribute significantly to the stability of RS-configured transition state. The mechanistic insights obtained in this study should open the door for the rational design of reactions for the effective construction of functional heterocyclic molecules via a sequential strategy with NHC organocatalysis.
中文翻译:

对NHC催化的级联迈克尔/羟醛/内酰胺化反应的见解:立体选择性的机理和起源†
理论上研究了N-杂环卡宾(NHC)催化的烯键和吲哚衍生的烯酮级联Michael /醛醇/内酰胺化反应的详细机理和立体选择性的起源。根据计算结果,该反应包括七个步骤,包括将NHC亲核加成至烯醛,分子内质子转移以形成Breslow中间体,吲哚衍生的α,β-不饱和酮与Breslow中间体,随后的[1,6]质子转移以提供烯醇化物中间体,分子内醇醛反应与质子转移过程,分子内内酰胺化反应以及NHC催化剂从产物中的释放。而且,优先生成RS配置产品。另外,NCI分析表明,更有效的C–H⋯π和π⋯π相互作用对RS配置的过渡态的稳定性有重要贡献。在这项研究中获得的机械见解应该打开用于官能杂环分子的有效的结构反应的合理设计的门通过与NHC有机催化顺序的战略。
更新日期:2018-05-14
中文翻译:

对NHC催化的级联迈克尔/羟醛/内酰胺化反应的见解:立体选择性的机理和起源†
理论上研究了N-杂环卡宾(NHC)催化的烯键和吲哚衍生的烯酮级联Michael /醛醇/内酰胺化反应的详细机理和立体选择性的起源。根据计算结果,该反应包括七个步骤,包括将NHC亲核加成至烯醛,分子内质子转移以形成Breslow中间体,吲哚衍生的α,β-不饱和酮与Breslow中间体,随后的[1,6]质子转移以提供烯醇化物中间体,分子内醇醛反应与质子转移过程,分子内内酰胺化反应以及NHC催化剂从产物中的释放。而且,优先生成RS配置产品。另外,NCI分析表明,更有效的C–H⋯π和π⋯π相互作用对RS配置的过渡态的稳定性有重要贡献。在这项研究中获得的机械见解应该打开用于官能杂环分子的有效的结构反应的合理设计的门通过与NHC有机催化顺序的战略。










































 京公网安备 11010802027423号
京公网安备 11010802027423号