当前位置:
X-MOL 学术
›
Asian J. Org. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Diels–Alder Reactions in Creating Complexity in Higher Order Isoprenoids: Proposed Biosynthesis and Biomimetic Total Syntheses
Asian Journal of Organic Chemistry ( IF 2.8 ) Pub Date : 2018-06-13 , DOI: 10.1002/ajoc.201800158 Vishnumaya Bisai 1 , Alakesh Bisai 2
Asian Journal of Organic Chemistry ( IF 2.8 ) Pub Date : 2018-06-13 , DOI: 10.1002/ajoc.201800158 Vishnumaya Bisai 1 , Alakesh Bisai 2
Affiliation
|
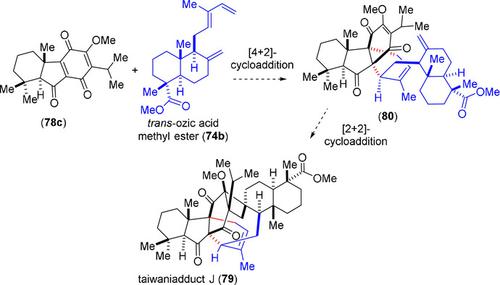
Diels–Alder reactions and hetero Diels–Alder reactions play crucial role in building complexity in many naturally occurring isoprenoids. A number of structurally intriguing complex isoprenoids have been isolated form Nature, those are believed to follow a [4+2]‐cycloaddition reaction in their biosynthetic proposal. In this focus review, we discuss on the recent efforts on biomimetic total syntheses of complex isoprenoids utilizing Diels–Alder and hetero Diels–Alder reactions. In particular, we have considered isoprenoids arising from a basic icetaxane, abietane, dinor‐abietane, and abeo‐abietane structural scaffolds.
中文翻译:

在高阶类异戊二烯中创造复杂性的Diels-Alder反应:拟议的生物合成和仿生总合成
Diels–Alder反应和杂Diels–Alder反应在许多天然存在的类异戊二烯的复杂性中起着至关重要的作用。从自然界中分离出了许多结构有趣的复杂类异戊二烯,据信它们在生物合成中遵循[4 + 2]-环加成反应。在这篇重点综述中,我们讨论了利用Diels-Alder反应和杂Diels-Alder反应进行复杂类异戊二烯仿生全合成的最新努力。特别是,我们已经考虑从基本icetaxane,松香烷,DINOR,松香烷产生异戊二烯和的abeo- -abietane结构支架。
更新日期:2018-06-13
中文翻译:

在高阶类异戊二烯中创造复杂性的Diels-Alder反应:拟议的生物合成和仿生总合成
Diels–Alder反应和杂Diels–Alder反应在许多天然存在的类异戊二烯的复杂性中起着至关重要的作用。从自然界中分离出了许多结构有趣的复杂类异戊二烯,据信它们在生物合成中遵循[4 + 2]-环加成反应。在这篇重点综述中,我们讨论了利用Diels-Alder反应和杂Diels-Alder反应进行复杂类异戊二烯仿生全合成的最新努力。特别是,我们已经考虑从基本icetaxane,松香烷,DINOR,松香烷产生异戊二烯和的abeo- -abietane结构支架。











































 京公网安备 11010802027423号
京公网安备 11010802027423号