当前位置:
X-MOL 学术
›
Asian J. Org. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
A Copper‐ and Phosphine‐Free Nickel(II)‐Catalyzed Method for C−H Bond Alkynylation of Benzothiazoles and Related Azoles
Asian Journal of Organic Chemistry ( IF 2.8 ) Pub Date : 2018-06-07 , DOI: 10.1002/ajoc.201800243 Ulhas N. Patel 1 , Benudhar Punji 1
Asian Journal of Organic Chemistry ( IF 2.8 ) Pub Date : 2018-06-07 , DOI: 10.1002/ajoc.201800243 Ulhas N. Patel 1 , Benudhar Punji 1
Affiliation
|
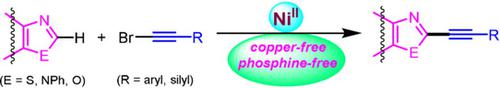
A phosphine‐free nickel(II)‐catalyzed method for the C(2)−H bond alkynylation of (benzo)thiazoles, (benz)imidazoles, and oxazoles is described. Well‐defined and air‐stable (Phen)NiCl2 catalyst efficiently catalyzes the coupling of diverse azoles with alkynyl bromides without the use of a copper co‐catalyst, and the method tolerates synthetically important functional groups. Preliminary mechanistic studies on this NiII‐catalyzed alkynylation emphasize the homogeneous nature of the catalyst, and rule out a radical manifold for the reaction. The synthetic utility of this Ni‐catalyzed method is demonstrated by further functionalizing the alkynylated benzothiazoles to 3‐methyl‐2‐(alkynyl)benzo[d]thiazolium salts that are known DNA cleaving agents.
中文翻译:

铜和无磷镍(II)催化方法联苯并噻唑和相关腈的C-H键炔化反应
描述了一种无膦的镍(II)催化方法,用于(苯并)噻唑,(苯并咪唑)和恶唑的C(2)-H键烷基化。定义明确且空气稳定的(Phen)NiCl 2催化剂可有效催化多种唑类与炔基溴的偶联,而无需使用铜助催化剂,该方法可耐受合成中重要的官能团。对该Ni II催化的炔基化反应的初步机理研究强调了催化剂的均相性质,并排除了反应的自由基。通过将炔基化的苯并噻唑进一步官能化为已知的DNA裂解剂3-甲基-2-(炔基)苯并[ d ]噻唑鎓盐,可以证明这种Ni催化方法的合成实用性。
更新日期:2018-06-07
中文翻译:

铜和无磷镍(II)催化方法联苯并噻唑和相关腈的C-H键炔化反应
描述了一种无膦的镍(II)催化方法,用于(苯并)噻唑,(苯并咪唑)和恶唑的C(2)-H键烷基化。定义明确且空气稳定的(Phen)NiCl 2催化剂可有效催化多种唑类与炔基溴的偶联,而无需使用铜助催化剂,该方法可耐受合成中重要的官能团。对该Ni II催化的炔基化反应的初步机理研究强调了催化剂的均相性质,并排除了反应的自由基。通过将炔基化的苯并噻唑进一步官能化为已知的DNA裂解剂3-甲基-2-(炔基)苯并[ d ]噻唑鎓盐,可以证明这种Ni催化方法的合成实用性。











































 京公网安备 11010802027423号
京公网安备 11010802027423号